Figure 2.
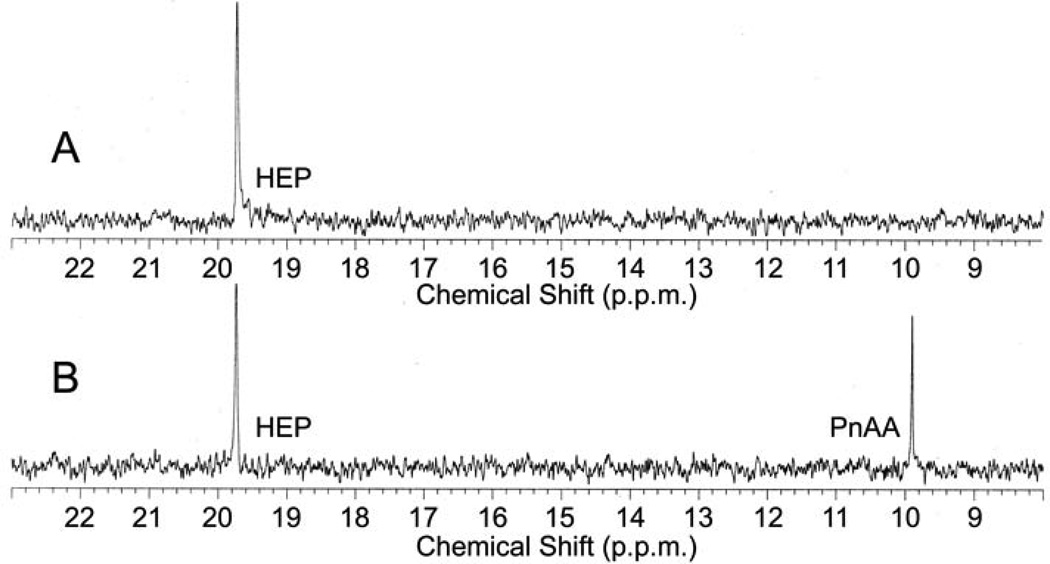
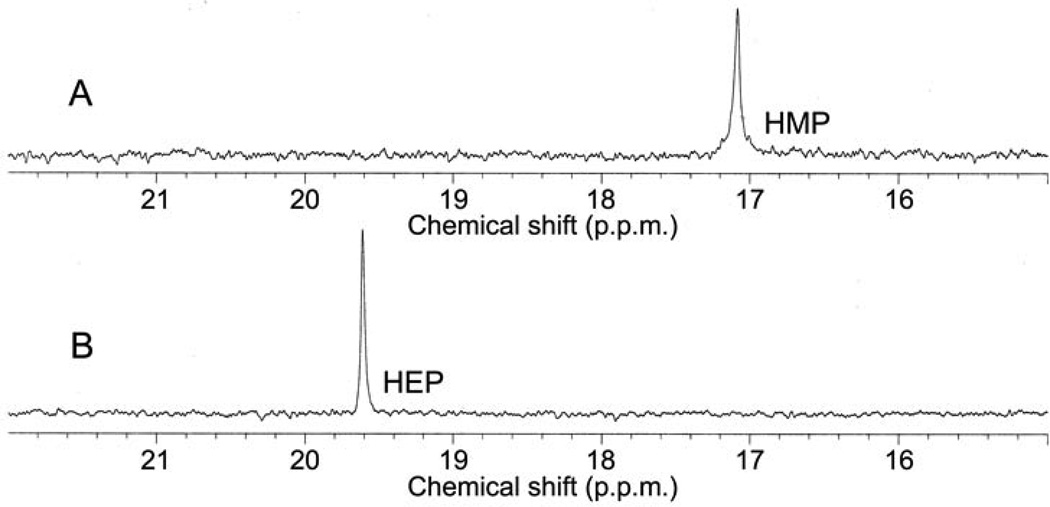
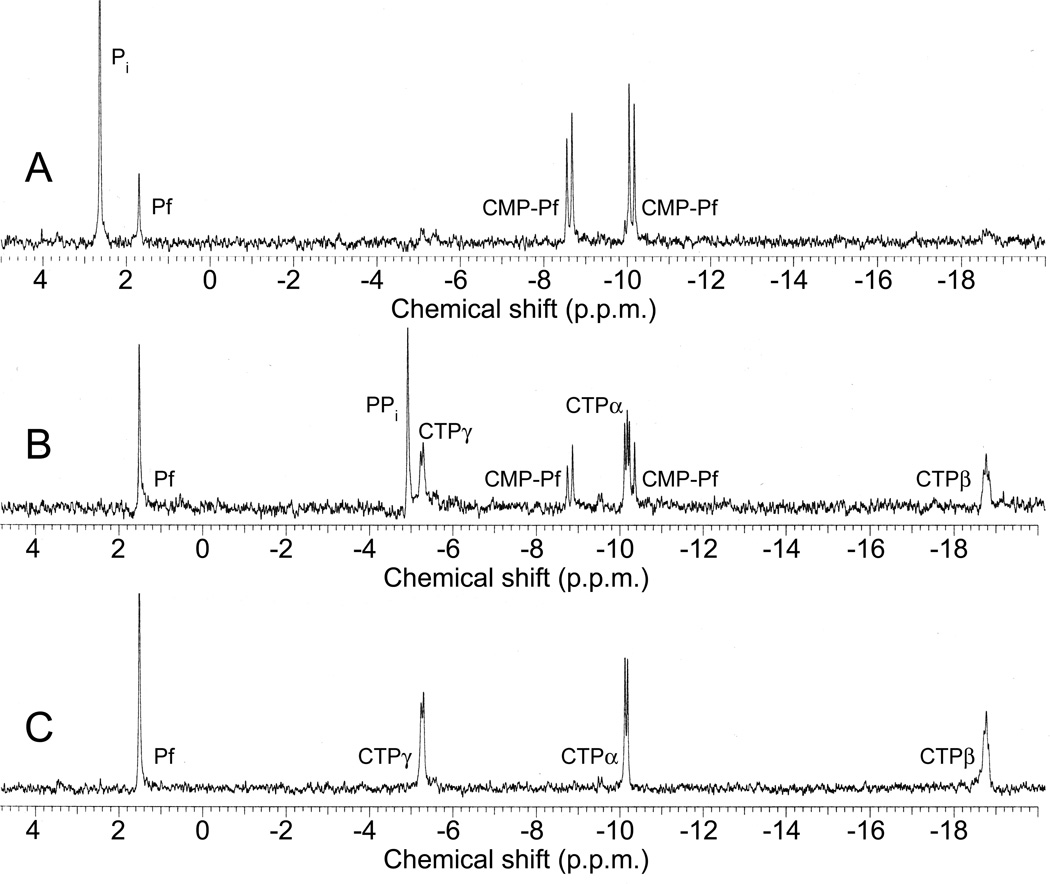
In vitro reconstitution of early PTT biosynthetic reactions monitored by 31P NMR. (a) 31P NMR results of the His-PhpC catalyzed reduction of phosphonoacetaldehyde (PnAA, δ 9.9) to hydroxyethylphosphonate (HEP, δ 9.7) in the presence of (A) NADH and (B), the same reaction performed in the presence of NADPH. (b) 31P NMR results confirming the direct in vitro conversion of hydroxyethylphosphonate to hydroxymethylphosphonate by PhpD. (A), an assay performed in the presence of Rosetta(DE3)pLysS/pJVD26 extract containing PhpD, showing the conversion of hydroxyethylphosphonate into hydroxymethylphosphonate (HMP, δ 17.1). (B) a control assay containing hydroxyethylphosphonate (HEP, δ 19.6) and Rosetta(DE3)pLysS extract. (c) 31P NMR results of assays establishing the CTP-phosphonoformate nucleotidyltransferase activity of His-PhpF. (A) A reaction containing His-PhpF, inorganic pyrophosphatase, CTP and phosphonoformate showing the accumulation of phosphate (δ 2.6), and the conversion of substrates into CMP-5’-phosphonoformate (δ −8.6, −10.1). The top of the phosphate peak is not shown to fit the spectrum to the figure. (B), the same reaction as in A in the absence of pyrophosphatase showing the partial conversion of phosphonoformate and CTP into CMP-5’-phosphonoformate (δ −8.6, −10.1) and pyrophosphate (δ −4.7) and (C) a control assay containing heat-inactivated His-PhpF, CTP (δ −5.1, −9.97, −18.6) and phosphonoformate (δ 1.7), The putative α, β, and γ designations of the CTP phosphorus nuclei have been assigned by comparison to those analogously found in ATP 30.