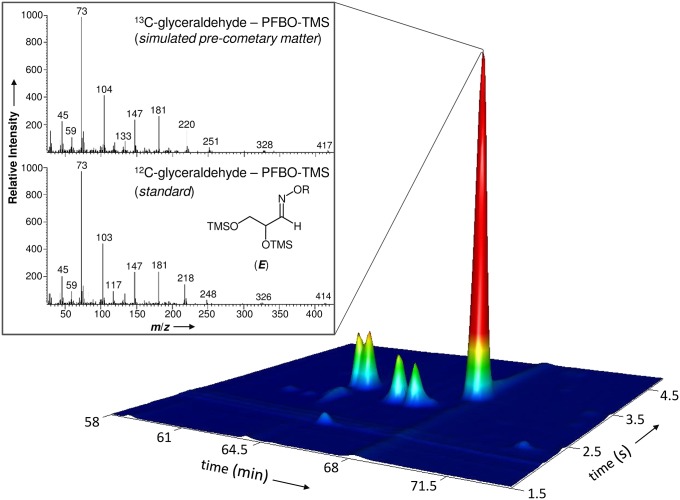
Fig. 2.
Glyceraldehyde detected in simulated precometary organic residues. Identification of glyceraldehyde as O-pentafluorobenzyl (R) oxime bis(trimethylsilyl) ether (PFBO-TMS) in laboratory organic residues using multidimensional gas chromatography. The corresponding external glyceraldehyde standard shows identical retention times and 12C isotopic signatures in its mass spectra. The mass fragmentation reveals that glyceraldehyde formed in the residue is entirely composed of 13C-isotopes provided by the 13CH3OH reactant present in the original ice mixture. Note that all aldehyde derivatives, except formaldehyde PFBO, can form syn (Z) and anti (E) stereoisomers of the oximes because the carbon nitrogen double bond (imine) formed during derivatization prevents rotation.