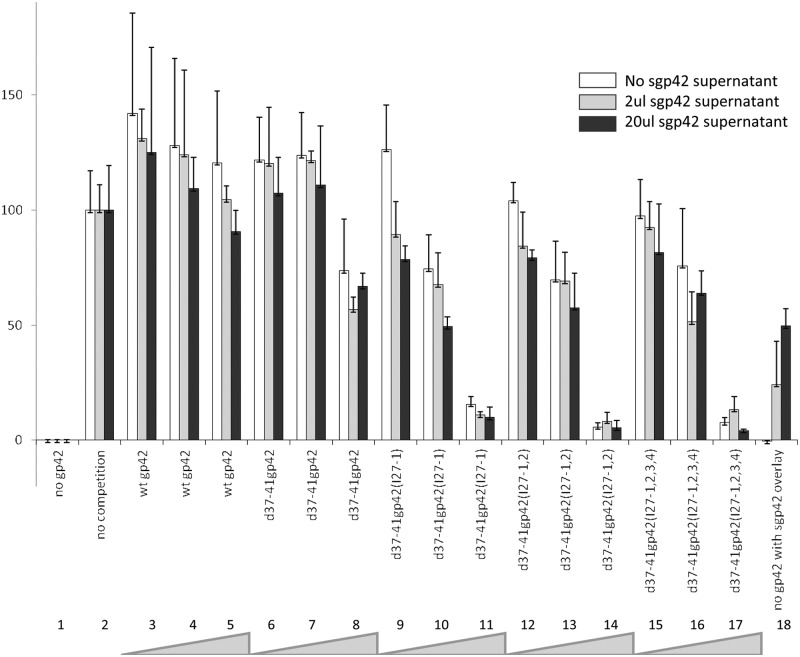
FIG 5 .
The gp42 linker mutants act in a dominant-negative fashion and compete with wild-type gp42 during B cell fusion. CHO-KI cells were transiently transfected with 0.5 µg of wild-type gp42 and increasing amounts of each of the gp42 linker mutants (0.01 µg, 0.10 µg, and 1.0 µg) indicated by the thickness of the gray wedge at the bottom of the figure in the presence of gH (0.5 µg), gL (0.5 µg), gB (0.5 µg), and T7 polymerase (0.8 µg). Twenty-four hours posttransfection, cells were detached and overlaid with Daudi B cells expressing HLA class II and T7 promoter. Zero, 2 or 20 µl of sgp42 supernatant was added at the time of overlay (white, gray, and black bars). Twenty hours after the overlay, fusion activity was assessed by the addition of passive lysis buffer, followed by the addition of luciferase substrate. Luciferase activity was measured with a PerkinElmer Victor plate reader. Data shown are results representative of the results of three independent experiments. Error bars represent standard deviations for the normalized values.