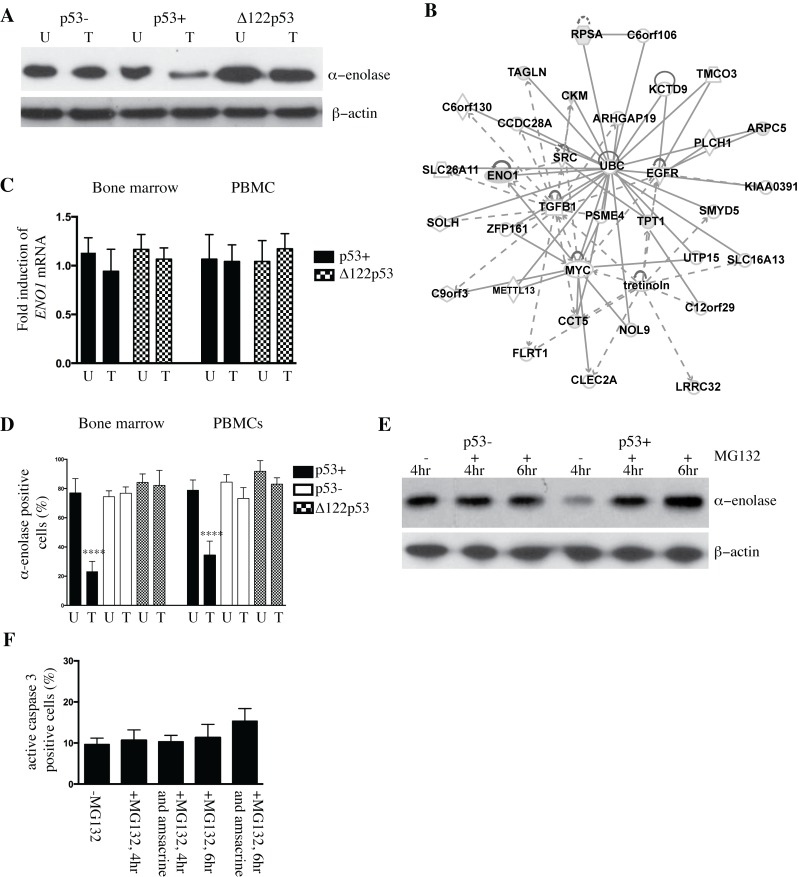
Figure 1. Altered abundance of alpha-enolase in p53+ and Δ122p53 bone marrow.
A. Alpha-enolase is decreased in wild-type p53 (p53+) bone marrow upon DNA damage with amsacrine treatment and increased in Δ122p53 bone marrow cells irrespective of amsacrine treatment compared with p53 null (p53-) cells by western blotting. Amsacrine treated (T) or untreated (U). B. Network analysis of the differentially expressed proteins in p53+ bone marrow untreated and p53+ bone marrow treated with amsacrine from two-dimensional fluorescence difference gel electrophoresis using Ingenuity Pathways Analysis software. Gray shading indicates proteins identified in the current study. Solid and dashed lines indicate direct and indirect interactions, respectively. C. The decrease in α-enolase in p53+ treated bone marrow was not due to reduced expression of ENO1. No significant differences were found in the amounts of ENO1 transcript expressed in p53+ and Δ122p53 bone marrow and peripheral blood mononuclear cells (PBMCs) in untreated cells (U) and cells treated with amsacrine (T) using real-time PCR. The results are expressed as the mean ± SD, from 3 mice per genotype and represent the fold increase in ENO1 expression, normalized for beta2-M expression. D. The percentage of alpha-enolase positive cells was reduced in p53+ bone marrow and peripheral blood mononuclear cells (PBMCs) treated with amsacrine. Bone marrow and PBMCs from five mice per genotype were extracted and treated with amsacrine (T) or left untreated (U). Following short-term culture, cells were fixed and cell clots sectioned. Alpha-enolase was detected using immunohistochemistry. Positive cells were identified by light microscopy and the percentage of positive cells per total cell count (500 cells) was compared between treated and untreated cells; the results are expressed as the mean ± SD (n = 6 mice per genotype), ****, P < 0.0001. E. The ubiquitin-associated proteasome inhibitor MG132 was added to p53+ and p53- bone marrow treated with amsacrine for 4 or 6 hours. Post-treatment, the amount of alpha-enolase in cell lysates was compared between cells treated with amsacrine or left untreated, by western blotting. F. MG132 treatment did not lead to a statistically significant increase in the percentage of apoptotic cells in p53+ bone marrow at 4 and 6 hours post-treatment with MG132 alone or with MG132 and amsacrine. Apoptotic cells were determined from counting the percentage of active caspase-3-positive cells in bone marrow from immunohistochemistry-stained sections and light microscopy. The percentage of positive cells per total cell count (500 cells) was compared between treated and untreated cells; the results are expressed as the mean ± SD (n = 3).