Abstract
Formononetin is a novel herbal isoflavonoid isolated from Astragalus membranaceus and possesses antitumorigenic properties. In the present study, we investigated the anti-proliferative effects of formononetin on human non-small cell lung cancer (NSCLC), and further elucidated the molecular mechanism underlying the anti-tumor property. MTT assay showed that formononetin treatment significantly inhibited the proliferation of two NSCLC cell lines including A549 and NCI-H23 in a time- and dose-dependent manner. Flow cytometric analysis demonstrated that formononetin induced G1-phase cell cycle arrest and promoted cell apoptosis in NSCLC cells. On the molecular level, we observed that exposure to formononetin altered the expression levels of cell cycle arrest-associated proteins p21, cyclin A and cyclin D1. Meanwhile, the apoptosis-related proteins cleaved caspase-3, bax and bcl-2 were also changed following treatment with formononetin. In addition, the expression level of p53 was dose-dependently upregulated after administration with formononetin. We also found that formononetin treatment increased the phosphorylation of p53 at Ser15 and Ser20 and enhances its transcriptional activity in a dose-dependent manner. Collectively, these results demonstrated that formononetin might be a potential chemopreventive drug for lung cancer therapy through induction of cell cycle arrest and apoptosis in NSCLC cells.
Keywords: Human non-small cell lung cancer, formononetin, proliferation, cell cycle, apoptosis
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, in which 80% of lung cancers are non-small cell lung cancer (NSCLC) with poor therapeutic efficacy when diagnosed [1,2]. Though there has been significant development in clinical treatment of NSCLC, its prognosis is still unsatisfactory due to the low rate of 5-year survival [3]. Fortunately, there are many extracts from traditional Chinese medicines showing better therapies to human NSCLC, leading us a new way to treat lung cancer in the future [4,5].
Traditional Chinese herbs are significant sources of drugs that serve as potential therapeutic compounds for cancer treatment. Astragalus membranaceus (Radix Astragali) has a long history of medicinal use in traditional Chinese medicine as an immunomodulating agent to treat diarrhea, anorexia and fatigue [6-8]. Recent studies have shown that Astragalus membranaceus can be used to alleviate the side-effects of cytotoxic antineoplastic drugs [6-8]. Formononetin is one of the major isoflavonoid constituents isolated from Astragalus membranaceus and demonstrates diverse pharmacological benefits. As a phytoestrogen, it exhibits a metabolic effect by upregulating interleukin-4 production in activated T cells via increased AP-1 DNA binding activity [11]. Formononetin also possesses antiinflammatory activity by inhibition of arachidonic acid release in HT-29 human colon cancer cells [12]. Accumulating evidences demonstrated the anticancer activity of formononetin on breast cancer [13], prostate cancer [14] and cervical cancer [15]. However, the inhibitory effect of formononetin on human lung cancer cells has never been investigated. Therefore, the present study aimed to explore the anti-proliferative effects of formononetin on lung cancer cells, and further elucidate the molecular mechanism underlying the anti-tumor property on human lung cancer.
Materials and methods
Reagents
Formononetin (purity > 99%) was purchased from Sigma (St. Louis, MO, USA)). Dulbecco’s modified Eagle’s medium (DMEM) culture medium, fetal bovine serum (FBS), phosphate-buffered saline (PBS), penicillin-streptomycin (PS) and 0.25% (w/v) trypsin/1 mM EDTA were purchased from Gibco (Grand Island, NY, USA).
Cell culture
The human NSCLC cell line A549, NCI-H23 and an immortalized human bronchial epithelial cell line 16HBE-T were purchased from the American Type Culture Collection (Rockville, MD) and cultured in DMEM supplemented with 10% FBS in an atmosphere containing 5% CO2 at 37°C.
MTT assay
Cell proliferation was determined by MTT assay. To be brief, A549 and NCI-H23 cells were seeded into 96-well plates at the density of 3 × 104 (cells/well) and left to adhere overnight. Cells were incubated with formononetin from 0~200 μM. Then 10 ml of 5 mg/ml MTT was added and incubated in dark at 37°C for 2 h. The absorbance was determined with the wavelength of 492 nm.
Cell cycle analysis
Cells were seeded at the density of 1.0 × 106 cells/well in a 6-well plate for 24 h, and then treated with formononetin. After 24 h, cells were washed twice with PBS, detached with trypsin and harvested. For cell cycle analysis, cells were harvested and collected by centrifugation, followed by fixation in ice-cold 70% ethanol at -20°C overnight. Then, cells were collected and stained with 100 μl PI staining solution for 30 min in the dark followed by cell cycle analysis.
Apoptosis detection
Apoptosis cells were detected with annexin V-FITC/PI according to the protocol of Annexin V-FITC cell Apoptosis Detection Kit (BD, USA). To be brief, A549 and NCI-H23 cells were seeded in a 6-well plate for 24 h and treated with different concentrations of formononetin. Cells were then harvested and washed twice with ice-cold PBS. Cells were then stained with annexin V-FITC and propidium iodide (PI) for 60 min in dark at room temperature in binding buffer. The cell apoptosis in A549 and NCI-H23 cells were detected by flow cytometry (FACSCalibur, USA).
Western blot analysis
A549 cells were treated with different concentrations of formononetin for 48 h. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to PVDF (Millipore, Bedford, MA, USA) membrane. The blots were blocked with 5% non-fat milk at room temperature for 1 h and incubated with the appropriate primary antibody including anti-p21, cyclin A, cyclin D1 (Cell Signaling, Beverly, MA); cleaved caspase-3, bax, bcl-2, p53, p-p53 Ser15/S20 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Then, the blots were incubated with peroxidase-conjugated secondary antibody. Bands were detected using western blot chemiluminescence reagent (Bio-Rad, Hercules, CA, USA).
Luciferase reporter assay
A549 cells were seeded into 24-well plates and cultured overnight. Then cells were transfected with pGL3-p53 firefly luciferase reporter plasmid and pRL-SV40 (Renilla luciferase) plasmid (Promega, USA) with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. 24 h post-transfection, the cells were treated with the indicated concentrations of formononetin for 24 h. Cell lysates were used for luciferase activity assay according to the Dual Luciferase Reporter Assay kit (Promega, USA) protocol.
Statistical analysis
Each experiment was performed in triplicate, and repeated at least three times. For statistical analysis, all the data were presented as means ± SD and treated for statistics analysis by SPSS program. Comparison between groups was made using ANOVA and statistically significant difference was defined as P < 0.05.
Results
Anti-proliferative effect of formononetin on NSCLC cells
A549 and NCI-H23 cells were exposed to different concentrations of formononetin (50, 100, 150 and 200 μM) for 12 h, 24 h and 48 h. MTT assay was performed to determine the effects of formononetin on NSCLC cells proliferation. We found that formononetin treatment significantly inhibited the proliferation of A549 and NCI-H23 cells in a time- and dose-dependent manner (Figure 1A and 1B). By contrast, formononetin had no obvious inhibitory effect on 16HBE-T cells (Figure 1C). These data demonstrated the anti-proliferative role of formononetin on NSCLC cells.
Figure 1.
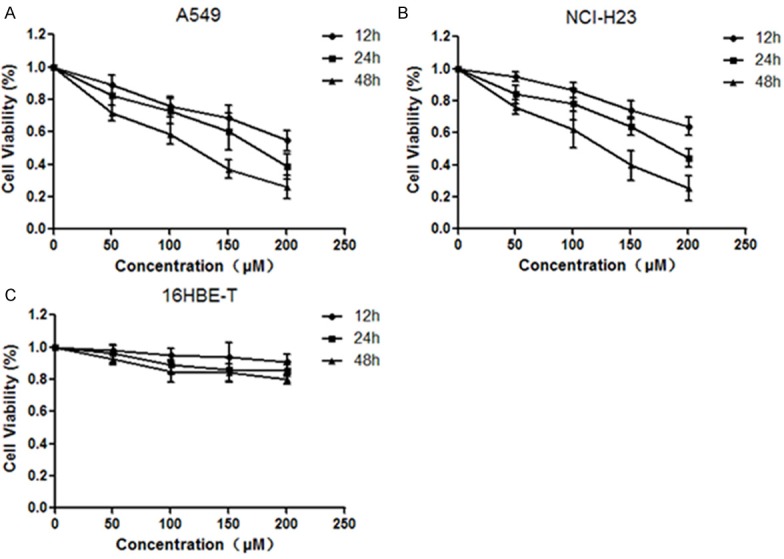
Inhibitory effects of formononetin on NSCLC cell proliferation. Lung cancer cells were treated with different concentrations of formononetin (50, 100, 150 and 200 μM) for 12 h, 24 h and 48 h. MTT assay was performed to determine the proliferation of A549 (A), NCI-H23 (B) and 16HBE-T cells (C).
Formononetin induced cell cycle arrest and promoted apoptosis in NSCLC cells
A549 and NCI-H23 cells were treated with different concentrations of formononetin (100, 150 and 200 μM) for 24 h and cell cycle distribution was analyzed by flow cytometry. Compared with the control group, administration of A549 cells with formononetin dose-dependently increased the proportions of cells in G1-phase, and decreased the number of cells in S phase (Figure 2A and 2B). Similar results were also observed in NCI-H23 cells after treatment with formononetin (Figure 2C and 2D). Moreover, the apoptosis rate of NSCLC cells was analyzed using Annexin V and PI staining. Compared with the control group, formononetin treatment significantly promoted the apoptosis rates of A549 (Figure 3A) and NCI-H23 (Figure 3B) cells in a concentration-dependent manner. Collectively, these results indicated that formononetin induced G1-phase cell cycle arrest and promoted apoptosis in NSCLC cells.
Figure 2.
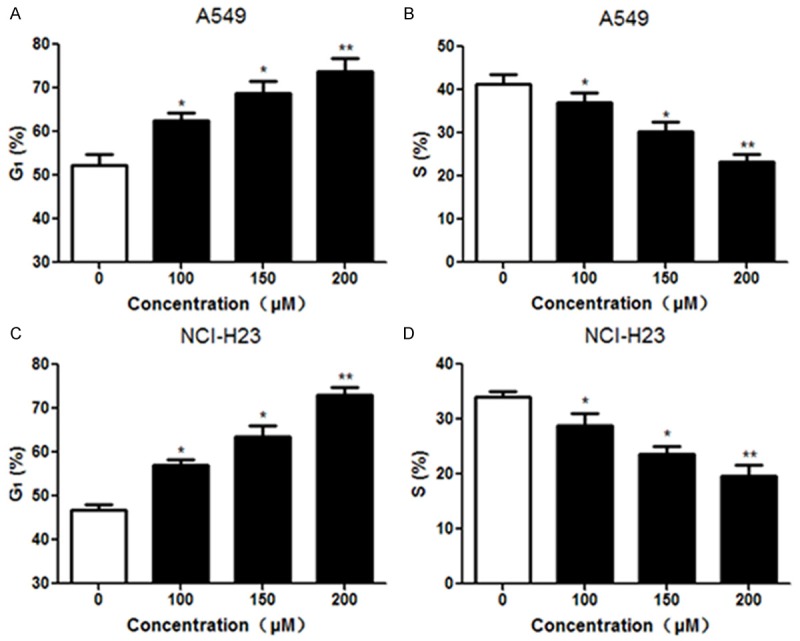
Formononetin induced cell cycle arrest in NSCLC cells. Lung cancer cells were treated with formononetin at indicated concentrations (100, 150 and 200 μM) for 24 h. The cell cycle distribution of A549 (A and B) and NCI-H23 (C and D) was analyzed by flow cytometry. *P < 0.05; **P < 0.01.
Figure 3.
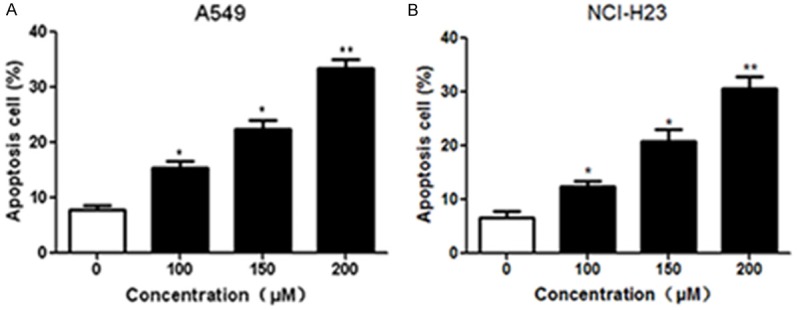
Formononetin promoted apoptosis in NSCLC cells, Lung cancer cells were treated with formononetin at indicated concentrations (100, 150 and 200 μM) for 24 h. Flow cytometry was performed to measure the apoptosis rate of A549 (A) and NCI-H23 (B) cells. *P < 0.05; **P < 0.01.
Formononetin mediates proteins expression related to cell cycle arrest and apoptosis
To further understand the molecular basis of formononetin-induced cell cycle arrest, we examined the expression levels of cell cycle-related proteins with western blot. A549 cells were treated with different concentrations of formononetin (100, 150 and 200 μM) for 48 h followed by western blot analysis (Figure 4A). We found that exposure to formononetin dose-dependently increased the protein expression of p21 (Figure 4B). The expression levels of the G1-phase cell cycle regulatory proteins including cyclin D1 (Figure 4C) and cyclin A (Figure 4D) were significantly decreased in a does-dependent manner after treatment with formononetin for 48 h.
Figure 4.
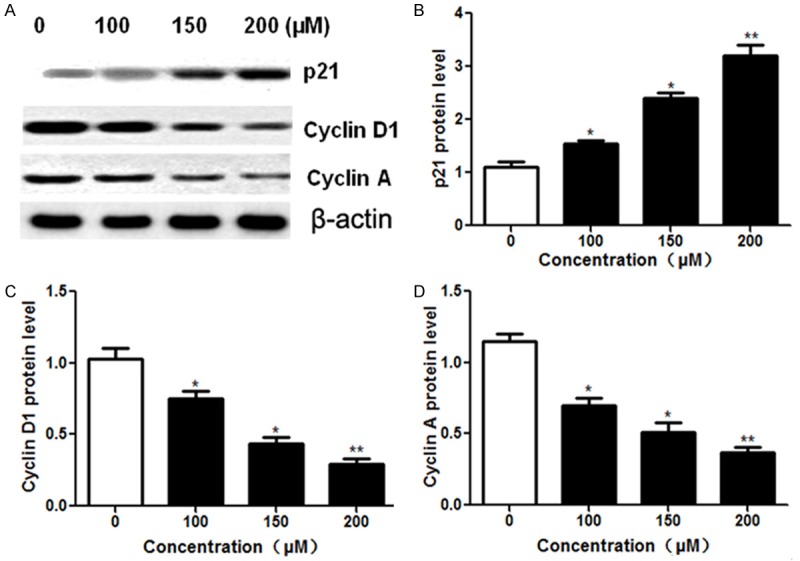
Effect of formononetin on the expression of cell cycle-related proteins. A549 cells were treated with formononetin at different concentrations (100, 150 and 200 μM) for 48 h. (A) Then protein expression was analyzed by western blot using antibodies against p21, cyclin D1 and cyclin A. β-actin was used as an internal control. The expression of p21 (B), cyclin D1 (C) and cyclin A (D) were measured by relative band intensities. *P < 0.05; **P < 0.01.
Next we determined the expression of apoptosis-related proteins in A549 cells after treatment with formononetin at indicated doses (Figure 5A). Considering that caspase activation often correlates with apoptosis, we detected the induction of cleavage of caspase-3 and observed an increased cleavage of caspase-3 in A549 cells in a dose-dependent manner (Figure 5B). In addition, we found that the pro-apoptotic protein bax was increased, whereas the anti-apoptotic protein bcl-2 was decreased in a dose-dependent fashion after formononetin treatment for 48 h (Figure 5C and 5D). Taken together, these results indicated that formononetin administration modulated the expression of critical cell cycle regulators and pro/anti-apoptotic signals, leading to cell cycle arrest and apoptosis in NSCLC cells.
Figure 5.
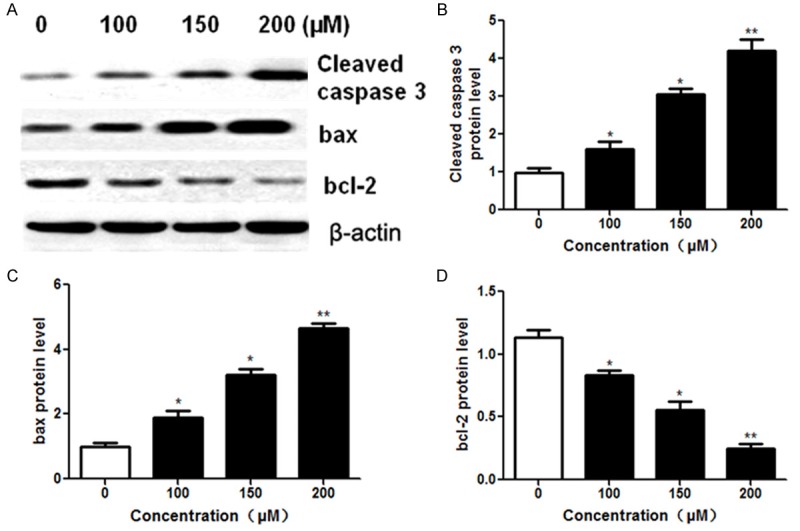
Effect of formononetin on the expression of apoptosis- related proteins. A549 cells were treated with formononetin at different concentrations (100, 150 and 200 μM) for 48 h. (A) Expression levels of apoptosis-related proteins were measured by using antibodies against Cleaved caspase 3, bax and bcl-2. β-actin was used as an internal control. Relative band intensities were used for quantification of Cleaved caspase 3 (B), bax (C) and bcl-2 (D). *P < 0.05; **P < 0.01.
p53 was pivotal in formononetin-induced cell apoptosis
p53, as a tumor suppressor, has been reported to be involved in cell apoptosis [16]. Therefore, we detected the protein expression of p53 in A549 cells to further explore the mechanism of formononetin’s anti-cancer effect. Results showed that the total p53 expression exhibited an increasing trend in response to formononetin treatment. We also demonstrated that the phosphorylation levels of p53 at Ser20 and Ser15 was significantly upregulated in a dose-dependent manner following formononetin treatment at different concentrations (Figure 6A and 6B). In addition, dual luciferase reporter assay indicated that the transactivation ability of p53 was significantly elevated following formononetin treatment (Figure 6C). These results suggested that p53 participated in formononetin’s effects on apoptotic induction in NSCLC cells.
Figure 6.
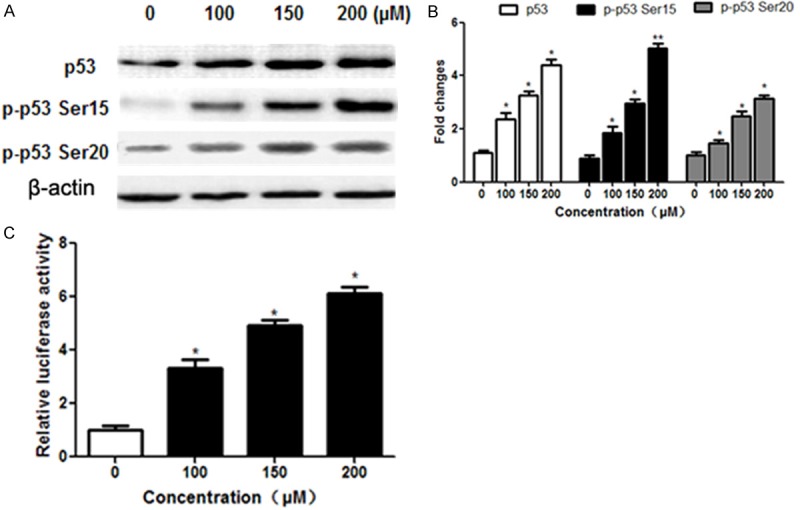
Formononetin promoted the activity of p53. A549 cells were treated with formononetin at different concentrations (100, 150 and 200 μM) for 48 h. A and B. Western blot was performed to determine the levels of p53 and p53 Ser15/20 phosphorylation. β-actin was used as an internal control. C. A549 cells were treated with the indicated concentrations of formononetin for 24 h and underwent dual luciferase reporter assays. *P < 0.05; **P < 0.01.
Discussion
Formononetin, a major isoflavone constituent of Radix Astragali, has been proposed to be potentially anticarcinogenic agent against several kinds of cancers such as liver cancer and breast cancer [12,17]. However, its effect on NSCLC cells and the underlying molecular mechanism have never been explored. Our study for the first time demonstrated that formononetin inhibited the NSCLC cells proliferation through induction of cell cycle arrest and apoptosis.
Firstly, we evaluated the anti-proliferative effect of formononetin on human lung cancer cells. MTT assay revealed that formononetin exhibited inhibitory effects on NSCLC cells lines including A549 and NCI-H23. However, no obvious effect on normal human bronchial epithelial cells was observed after treatment with formononetin. Taken together, these results showed that formononetin inhibited the proliferation of lung cancer cells.
Cell cycle is a precise process controlled by cell cycle checkpoints which guarantee the fidelity of cell division. Cell cycle arrest could be triggered by exogenous/endogenous stimulating factors and resulted in breakdown of cell division, cell death and apoptosis [18-20]. Previous study found that formononetin treatment decreased cyclin D1 mRNA and protein expression, thus inducing cell cycle arrest in human breast cancer cells [21]. In present study, we found that treatment with formononetin for 24 h induced cell cycle arrest in G1-phase. More importantly, we found that treatment of formononetin for 24 h dose-dependently increased the protein expression of p21, and decreased the expression levels of cell cycle regulatory proteins such as cyclin A and cyclin D1.
Apoptosis is the process of programmed cell death which is characterized by typical cellular and molecular features such as cell shrinkage, externalization of phosphatidylserine and condensation of chromatin [22,23]. Multiple studies confirmed the therapeutic effect of formononetin on cancer treatment through induction of cell apoptosis and critical pro-apoptotic proteins expression [24,25]. We demonstrated that formononetin exposure for 24 h substantially increased cell apoptosis in a dose-dependent manner. On the molecular level, formononetin administration promoted the cleavage of caspase-3 and expression of the pro-apoptotic protein bax. Meanwhile, the anti-apoptotic protein bcl-2 was significantly decreased in a does-dependent manner after treatment with formononetin. Collectively, these above results suggested that formononetin’s inhibitory effect on cell growth acted through inducing cell cycle arrest and apoptosis.
The p53 tumor suppressor plays a crucial role in multi-cellular organisms, where it regulates cell apoptosis and cell cycle [26,27]. Thus, it is a tempting strategy for cancer therapy to reverse downregulation of p53 in cancer cells. Intriguingly, we found that formononetin-induced apoptosis in A549 cells was mediated by accumulation of p53 protein expression. As an important transcriptional factor, the activity of p53 is mainly regulated by post-translational modifications including phosphorylation, acetylation and ubiquitination [28-30]. Previous studies have demonstrated that the phosphorylation of p53 at Ser15 or Ser20 could increase p53 stability and promote cell apoptosis [31,32]. In our study, formononetin treatment upregulated the phosphorylation levels of p53 at Ser20 and Ser15 in a dose-dependent manner. Moreover, dual luciferase assay showed that the transactional ability of p53 was significantly enhanced after formononetin administration. These results suggested that p53 participated in formononetin’s effect on apoptotic induction in NSCLC cells.
In conclusion, our study demonstrated that formononetin inhibited the proliferation of lung cancer cells through induction of cell cycle arrest and apoptosis. Thus, our study provides a new mechanism to explain the chemopreventive effect of formononetin on cancer and suggests that formononetin might be a new potential medicine that could be used for lung cancer therapy.
Acknowledgements
This study was supported by Science & Technology Fund Planning Project of Shanghai Chest Hospital (2014YZDC10600).
Disclosure of conflict of interest
None.
References
- 1.Kratz JR, He J, Van Den Eeden SK, Zhu ZH, Gao W, Pham PT, Mulvihill MS, Ziaei F, Zhang H, Su B, Zhi X, Quesenberry CP, Habel LA, Deng Q, Wang Z, Zhou J, Li H, Huang MC, Yeh CC, Segal MR, Ray MR, Jones KD, Raz DJ, Xu Z, Jahan TM, Berryman D, He B, Mann MJ, Jablons DM. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: Development and international validation studies. Lancet. 2012;379:823–832. doi: 10.1016/S0140-6736(11)61941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy N. Lung cancer: Gastric giveaways. Nat Rev Cancer. 2013;13:291. doi: 10.1038/nrc3514. [DOI] [PubMed] [Google Scholar]
- 3.Gregory DL, Hicks RJ, Hogg A, Binns DS, Shum PL, Milner A, Link E, Ball DL, Mac MM. Effect of PET/CT on management of patients with non-small cell lung cancer: Results of a prospective study with 5-year survival data. J Nucl Med. 2012;53:1007–1015. doi: 10.2967/jnumed.111.099713. [DOI] [PubMed] [Google Scholar]
- 4.He H, Zhou X, Wang Q, Zhao Y. Does the course of astragalus-containing Chinese herbal prescriptions and radiotherapy benefit to non-small-cell lung cancer treatment: A meta-analysis of randomized trials. Evid Based Complement Alternat Med. 2013;2013:426207. doi: 10.1155/2013/426207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lev-Ari S, Starr A, Katzburg S, Berkovich L, Rimmon A, Ben-Yosef R, Vexler A, Ron I, Earon G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J Nutr Biochem. 2014;25:843–850. doi: 10.1016/j.jnutbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Xuan X, Li M, Gao P, Zheng Y, Zang W, Zhao G. Astragalus saponins affects proliferation, invasion and apoptosis of gastric cancer BGC-823 cells. Diagn Pathol. 2013;8:179. doi: 10.1186/1746-1596-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Cho WC, Leung KN. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol. 2007;113:132–141. doi: 10.1016/j.jep.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Huang LH, Yan QJ, Kopparapu NK, Jiang ZQ, Sun Y. Astragalus membranaceus lectin (AML) induces caspase-dependent apoptosis in human leukemia cells. Cell Prolif. 2012;45:15–21. doi: 10.1111/j.1365-2184.2011.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo L, Bai SP, Zhao L, Wang XH. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: Effects on quality of life and survival. Med Oncol. 2012;29:1656–1662. doi: 10.1007/s12032-011-0068-9. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Kim SH, Cho D, Kim TS. Formononetin, a phyto-oestrogen, and its metabolites up-regulate interleukin-4 production in activated T cells via increased AP-1 DNA binding activity. Immunology. 2005;116:71–81. doi: 10.1111/j.1365-2567.2005.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang MH, Kim J, Khan IA, Walker LA, Khan SI. Nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) modulators from natural products as anti-cancer agents. Life Sci. 2014;100:75–84. doi: 10.1016/j.lfs.2014.01.075. [DOI] [PubMed] [Google Scholar]
- 13.Zhou R, Xu L, Ye M, Liao M, Du H, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753–60. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 14.Liu XJ, Li YQ, Chen QY, Xiao SJ, Zeng SE. Up-regulating of RASD1 and apoptosis of DU-145 human prostate cancer cells induced by formononetin in vitro. Asian Pac J Cancer Prev. 2014;15:2835–2839. doi: 10.7314/apjcp.2014.15.6.2835. [DOI] [PubMed] [Google Scholar]
- 15.Jin YM, Xu TM, Zhao YH, Wang YC, Cui M. In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HeLa. Tumour Biol. 2014;35:2279–2284. doi: 10.1007/s13277-013-1302-1. [DOI] [PubMed] [Google Scholar]
- 16.Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, Tighiouart M, Khuri FR, Chen ZG, Shin DM. Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: Potential role of p53. J Biol Chem. 2010;285:34557–34565. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansoor TA, Ramalho RM, Luo X, Ramalhete C, Rodrigues CM, Ferreira MJ. Isoflavones as apoptosis inducers in human hepatoma HuH-7 cells. Phytother Res. 2011;25:1819–1824. doi: 10.1002/ptr.3498. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Ma W, Zheng W. Deguelin, a novel antitumorigenic agent targeting apoptosis, cell cycle arrest and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol. 2013;1:215–219. doi: 10.3892/mco.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P, Chang C, Wang H. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature. 2007;449:1068–1072. doi: 10.1038/nature06256. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Zeng J, Xin M, Huang W, Chen X. Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res. 2011;43:681–686. doi: 10.1055/s-0031-1286306. [DOI] [PubMed] [Google Scholar]
- 22.Soriano ME, Scorrano L. Traveling Bax and forth from mitochondria to control apoptosis. Cell. 2011;145:15–17. doi: 10.1016/j.cell.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen W, Zhu F, Zhang J, Keum YS, Zykova T, Yao K, Peng C, Zheng D, Cho YY, Ma WY, Bode AM, Dong Z. MST1 promotes apoptosis through phosphorylation of histone H2AX. J Biol Chem. 2010;285:39108–39116. doi: 10.1074/jbc.M110.151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Bi L, Ye Y, Chen J. Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr Cancer. 2014;66:656–661. doi: 10.1080/01635581.2014.894098. [DOI] [PubMed] [Google Scholar]
- 25.Huang WJ, Bi LY, Li ZZ, Zhang X, Ye Y. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm Biol. 2013 doi: 10.3109/13880209.2013.842600. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Goh AM, Coffill CR, Lane DP. The role of mutant p53 in human cancer. J Pathol. 2011;223:116–126. doi: 10.1002/path.2784. [DOI] [PubMed] [Google Scholar]
- 27.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin a induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Hu H, Malewicz B, Wang Z, Lu J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004;3:877–884. [PubMed] [Google Scholar]
- 29.Kim JH, Yoon EK, Chung HJ, Park SY, Hong KM, Lee CH, Lee YS, Choi K, Yang Y, Kim K, Kim IH. P53 acetylation enhances Taxol-induced apoptosis in human cancer cells. Apoptosis. 2013;18:110–120. doi: 10.1007/s10495-012-0772-8. [DOI] [PubMed] [Google Scholar]
- 30.Michiue H, Tomizawa K, Matsushita M, Tamiya T, Lu YF, Ichikawa T, Date I, Matsui H. Ubiquitination-resistant p53 protein transduction therapy facilitates anti-cancer effect on the growth of human malignant glioma cells. Febs Lett. 2005;579:3965–3969. doi: 10.1016/j.febslet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Madan E, Gogna R, Pati U. P53 Ser15 phosphorylation disrupts the p53-RPA70 complex and induces RPA70-mediated DNA repair in hypoxia. Biochem J. 2012;443:811–820. doi: 10.1042/BJ20111627. [DOI] [PubMed] [Google Scholar]
- 32.Safa M, Kazemi A, Zand H, Azarkeivan A, Zaker F, Hayat P. Inhibitory role of cAMP on doxorubicin-induced apoptosis in pre-B ALL cells through dephosphorylation of p53 serine residues. Apoptosis. 2010;15:196–203. doi: 10.1007/s10495-009-0417-8. [DOI] [PubMed] [Google Scholar]


