Abstract
Previous research has demonstrated that chronic cigarette smoking and major depressive disorder (MDD) are each associated with cognitive decrements. Further, these conditions co-occur commonly, though mechanisms in the comorbid condition are poorly understood. There may be distinct, additive, or overlapping factors underlying comorbid cigarette smoking and MDD. The present study investigated the impact of smoking and MDD on executive function and emotion processing. Participants (N=198) were grouped by diagnostic category (MDD and healthy controls, HC) and smoking status (ever-smokers, ES and never-smokers, NS). Participants completed the Facial Emotion Perception Test (FEPT), a measure of emotional processing, and the parametric Go/No-go task (PGNG), a measure of executive function. FEPT performance was analyzed using ANCOVA with accuracy and reaction time as separate dependent variables. Repeated measures MANCOVA was conducted for PGNG with performance measure and task level as dependent variables. Analyses for each task included diagnostic and smoking group as independent variables, and gender was controlled for. Results for FEPT reveal lower overall accuracy was found for ES relative to NS, though MDD did not differ from HC. Post-hoc analyses revealed ES were poorer at identifying happy and sad, but not fearful or angry, faces. For PGNG, poorer performance was observed in MDD relative to HC in response time to Go targets, but there were no differences for ES and NS. Interaction of diagnosis and smoking group was not observed for performance on either task. The results of this study provide preliminary evidence for distinctive cognitive decrements in smokers and individuals with depression.
Keywords: smoking, depression, executive functioning, attention, emotion processing, facial affect perception, ex-smokers
1.1 Introduction
Chronic cigarette smoking is linked to several adverse health outcomes, including cerebrovascular disease, cardiovascular disease, respiratory disease, cancer and major depressive disorder (MDD). Such diseases accounted for two-thirds of deaths worldwide between the years 1990 and 2010 (Lozano, et al., 2012). Previous research suggests that annual rates of morbidity and mortality are greater for cigarette smokers compared to nonsmokers (McGinnis & Foege, 1993), as well as for individuals with MDD compared to those without MDD (Wulsin, Vaillant, & Wells, 1999). Further, rates of smoking are higher among individuals with MDD (Dierker, Avenevoli, Stolar, & Merikangas, 2002; Langenecker, et al., 2009). Trosclair and Dube (2010) found that 33% of individuals with lifetime history of depression or anxiety were current smokers. Likewise, 22% of individuals without psychiatric illness have a history of smoking (Pratt & Brody, 2010). Also, depressive symptoms are heightened during smoking cessation attempts, and may interfere with smoking cessation attempts (Covey et al., 1990; Glassman et al., 1990; Tsoh & Sharon, 2004; Langenecker, et al., 2009). Despite the substantial overlap of cigarette smoking and MDD, the co-occurrence of these conditions remains understudied.
Prior explorations have implicated genetics, negative affectivity, attentional dysfunction, and disrupted nicotinergic function as overlapping, distinct, and additive factors in comorbid MDD and smoking (Gilbert & Gilbert, 1995; Grant, Hasin, Chou, Stinson, & Dawson, 2004; Spada, Nikcevic, Moneta, & Wells, 2007; Tsuang, Francis, Minor, Thomas, & Stone, 2012). Further, both smoking (Gilbert & Gilbert, 1995; Carmody, Vieten, & Astin, 2007; Durazzo, Meyerhoff, & Nixon, 2010) and MDD (Bourke, Douglas, & Porter, 2010; Iverson, Brooks, Langenecker, & Young, 2011; Langenecker, et al., 2005) have independently been associated with affective and cognitive impairments known to detrimentally impact functioning in a variety of settings. For instance, emotion perception, the ability to identify and respond to facial expressions of emotion, is critical for successful social interactions. Difficulties with emotion perception can result in interpersonal communication problems that can impair an individual’s social and vocational practices (Bourke, Douglas, & Porter, 2010; Carton, Kessler, & Pape, 1999; Langenecker, et al., 2005). Likewise, deficits in executive functions, which include abilities to plan, make decisions, attend to stimuli, and inhibit inappropriate responses, may hinder one’s ability to function optimally. Ultimately, emotion processing and executive function decrements may interfere with recovery from depressive episodes (Porter, Bourke, & Gallagher, 2007) or an individual’s ability to quit smoking (Mendrek, et al., 2006; Carmody, Vieten, & Astin, 2007). In MDD, executive dysfunction is associated with poor treatment response to standard depression treatments (Kampf-Sherf, et al., 2004). Thus, obtaining critical information about the impact of smoking on executive functions and emotional processing among individuals with and without MDD may inform clinical care by guiding existing interventions for these conditions, while also directing the development of novel treatments. For example, if executive functioning deficits confer risk for smoking and MDD together, but not MDD alone, then treatment decisions that target executive functioning may be ineffective in MDD alone.
Despite the advantages of exploring affective and executive functions in comorbid MDD and cigarette smoking, there is little research on the topic. Typically, smoking precludes participation in neuropsychological studies of MDD, and MDD is often an exclusionary, uncontrolled, or self-report variable in studies of smoking. While exclusion as a methodology serves to elucidate the specific associations of each condition with cognition independently, the findings from these studies may have limited ecological utility given high rates of comorbidity. Comorbid MD and smoking may have shared risk factors, such as disruption of executive functions and emotion perception.
Executive impairments are among the most common cognitive symptoms associated with both MDD (Porter, Bourke, & Gallagher, 2007; Rogers, et al., 2004) and cigarette smoking (Jacobsen, et al., 2005; Mendrek, et al., 2006; Swan & Lessov-Schlaggar, 2007). Impairments in attention and executive functioning appear to persist following the remission of depressive episodes and are thought to represent trait characteristics or risk factors for depression (Langenecker, Lee, & Bieliauskas, 2009; Paelecke-Habermann, Pohl, & Leplow, 20005). It is less clear, however, whether executive dysfunction continues after individuals have quit smoking. Existing studies of executive functions in temporarily abstinent smokers reveal behavioral decrements similar to those observed in MDD, yet this does not address the long-term effects of smoking (Swan & Lessov-Schlaggar, 2007). Among chronic smokers, cognitive decrements include reduced psychomotor speed, cognitive flexibility, and visual search speed (Kalmijn, van Boxtel, Verschuren, Jolles, & Launer, 2002; Richards, Jarvis, Thompson, & Wadsworth, 2003; Durazzo, Meyerhoff, & Nixon, 2010), though reports are inconsistent as samples and methodology vary greatly across studies. Findings on executive functions in former smokers relative to never smokers are also mixed, although one study did report that those who were able to quit had better executive functioning (Ernst, Heishman, Spurgeon, & London, 2001).
MDD often presents with emotional processing impairments (Gollan, McCloskey, Hoxha, & Coccaro, 2010; Versace, et al., 2010). For smoking, research suggests that negative affect is a motivation for smoking in a larger percentage of smokers (Kassel, Stroud & Paronis, 2003). Cognitive theories of psychiatric conditions such as depression propose that biases in judgment of emotional processes are disease-related. When such biases occur, individuals misinterpret situations and may respond in a maladaptive manner, which further exacerbates their conditions (Kahler, et al., 2012). MDD has been associated with impaired recognition of facial expressions (Bourke, Douglas, & Porter, 2010; Wright, et al., 2009; Kohler, Hoffman, Eastman, Healey, & Moberg, 2011). It has been suggested that some of these impairments in emotion perception resolve during remission and that increased bias of judging ambiguous faces as negative facial emotions may be predictive of persistent depression at later time points (Hale, 1998; Bouhuys, Geerts, & Gordijn, 1999). In smoking, it is unclear whether the pattern of poor emotion identification observed for MDD is also present in smokers.
As a means of addressing limitations in the literature, the present study will examine shared mechanisms and impact of MDD and smoking upon emotion perception and executive functioning. It was expected that individuals with MDD and a history of cigarette smoking would perform worse than non-depressed, non-smoking controls on tests of executive function, examining attention and inhibitory control, and emotion identification. Exploratory analyses addressed the impact of comorbid smoking and MDD on measures of executive functioning and emotion identification.
2.1 Method
2.1.1 Participants
This was a retrospective study of 194 participants (78 healthy controls, HC, and 116 MDD). Depressed participants completed smoking and cognitive measures as part of a standard intake battery prior to their first psychiatric assessment for depression at the University of Michigan Depression Center. HC were recruited through projects at the University of Michigan as part of a larger sample reported elsewhere (Langenecker, et al., 2007). Absence of psychiatric conditions in HC were confirmed for 13 participants using the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, and Gibbon, 1995) non-patient version and for 65 participants using the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994). Key demographic and clinical variables relevant to diagnosis group and smoking status are reported in Table 1.
Table 1.
Participant Characteristics
| HC | MDD | |||
|---|---|---|---|---|
| NS (n=63) | ES (n=15) | NS (n=72) | ES (n=44) | |
| Age | 33.54 (12.93) | 33.67 (12.87) | 36.58 (11.67) | 35.93 (13.51) |
| Gender (% female) | 52% | 68% | 73% | 61% |
| Education* | 15.35 (2.35) | 13.93 (1.98) | 15.53 (2.71) | 14.91 (2.62) |
| IQ | 106.69 (6.41) | 101.80 (11.46) | 105.07 (7.97) | 104.95 (7.94) |
| PHQ** | 2.14 (3.82) | 1.67 (2.40) | 15.35 (5.79) | 15.26 (5.46) |
| Age of Onset of Depression | -- | -- | 23.27 (10.60) | 21.84 (12.99) |
| % Family History of Depression*** | 0.2% | 0% | 74% | 57% |
| % Family History of Other Mental Illness**** | 2% | 30% | 88% | 73% |
| % Current Smokers | -- | 53% | -- | 64% |
| Age of Onset-Smoking | -- | 15.11 (1.54) | -- | 18.13 (4.79) |
| Years Smoked | -- | 13.00 (12.82) | -- | 13.48 (11.01) |
| Number of Cigarettes per Day | -- | 11.07 (7.39) | -- | 11.69 (7.08) |
| FTND Total***** | -- | 1.14 (1.79) | -- | 3.05 (3.57) |
| (At Highest Use) | 4.86 (2.67) | 5.00 (3.04) | ||
Note: ES, ever smokers; NS, never smokers; PHQ, Patient Health Questionnaire; FTND, Fagerstrom Test of Nicotine Dependence; --, not applicable. PHQ-9 was used for HC; PHQ-8 was used for MDD;
ES < NS on education, p < .05;
HC < MDD on PHQ scores, p < .01;
for % of participants with at least 1 family member with depression, MDD, NS > MDD, ES > HC, NS and ES, p < .001;
for % of participants with at least 1 family member with depression, MDD, NS > MDD, ES > HC, ES > HC, ES, p < .05;
MDD > HC on FTND total, p < .05.
MDD participants were diagnosed via clinical interview by board-certified psychiatrists (n = 93), with the SCID-IV patient version (n = 15), or with the DIGS (n = 8). Among MDD, most participants met criteria for diagnosis of MDD alone (71%) or MDD comorbid with an anxiety disorder (12%). The remaining MDD subjects had a diagnosis within the broader spectrum of mood disorders (e.g., mood disorder NOS (9%), dysthymia (0.2%) or no DSM-IV diagnosis (7.8%, yet with significant depression symptoms). There were 64% of MDD who were currently taking psychotropic medications (mean number of medications = 1.39, SD = 1.49). No HC participants met criteria for any psychiatric disorder (current or past). Among MDD and HC, there were 135 never smokers (NS) and 59 former and current smokers (ever smokers, ES). ES status was identified by Fagerstrom Test for Nicotine Dependence and Smoking History Questionnaire for clinical patients, and by FTND plus SCID or DIGS for other patients. Among ES, 36 participants were current smokers and 23 participants were current smokers. Approval for waiver of informed consent was given by the University of Michigan Medical School Institutional Review Board to retrospectively access and de-identify clinical data for research purposes.
2.1.2 Measures
Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991)
A six item self-report instrument designed to assess degree of tobacco dependence, the FTND has extensive use in the field of nicotine and tobacco research (Weinberger, et al., 2007; Charbol, Niezborala, Chastan, & de Leon, 2005). It has demonstrated validity for both current and retrospective assessment of tobacco use (Suchanek Hudmon, Pomerleau, Brigham, Javitz, & Swan, 2005).
Smoking History Questionnaire (SHQ; Brown, Lejuez, Kahler, & Strong, 2002)
A select subset of questions from the SHQ, a brief smoking history questionnaire, included eight items on smoking status, smoking rate, years smoked, when last smoked, desire to quit and use of other tobacco products was administered.
Patient Health Questionnaire-9 (PHQ-9; Kroenke, Spitzer, & Williams, 2001)
The PHQ-9 is a nine-item Likert questionnaire used to assess the DSM-IV symptoms of depression. Individuals rate the frequency of each item over the preceding two weeks on a scale from 0 (“not at all”) to 3 (“nearly every day”). Total scores of 10 or greater indicate at least moderate levels of depression. The PHQ-8 excludes the item of the PHQ-9 regarding suicidal ideation, and has been suggested for use in research settings where further probing is not feasible (Kroenke & Spitzer, 2002). Both measures have been successfully used by our group and others (Wright, et al., 2009; Huang, Chung, Kroenke, Delucchi, & Spitzer, 2006).
The Synonym Knowledge task (SKT; based on the Shipley Institute of Living Scale, Shipley, 1946)
The SKT, a 40-item measure, was used as an estimate of verbal intelligence. It was expected that this would serve as a control task, with no differences between the groups. Participants were presented with a word and then asked to choose which of four words was most similar in meaning to the word first presented (Langenecker, et al., 2007).
The Facial Emotion Perception task (FEPT; Langenecker, et al., 2005; Rapport, Friedman, Tzelepis, & VanVoorhis, 2002)
The FEPT is a seven minute task that was used to assess accuracy and speed of recognition of facial expressions (e.g., impaired emotion perception), an area of impaired functioning in depression and other mood disorders research (LeDoux, 2000; Gur, et al., 1992). Participants were presented with and asked to rapidly categorize faces (Ekman and Friesen, 1976) and animals (control condition). For the face trials, participants categorized the facial expression into one of four possibilities: happy, sad, angry, or fearful. For the animal trials, participants categorized the animal into one of four possibilities: dog, cat, primate, or bird. A stimulus is presented for 300 ms, followed a by a mask for 100 ms, and then 2600 ms are provided as a response window. Trials are separated by the presentation of a cross for 500 ms.
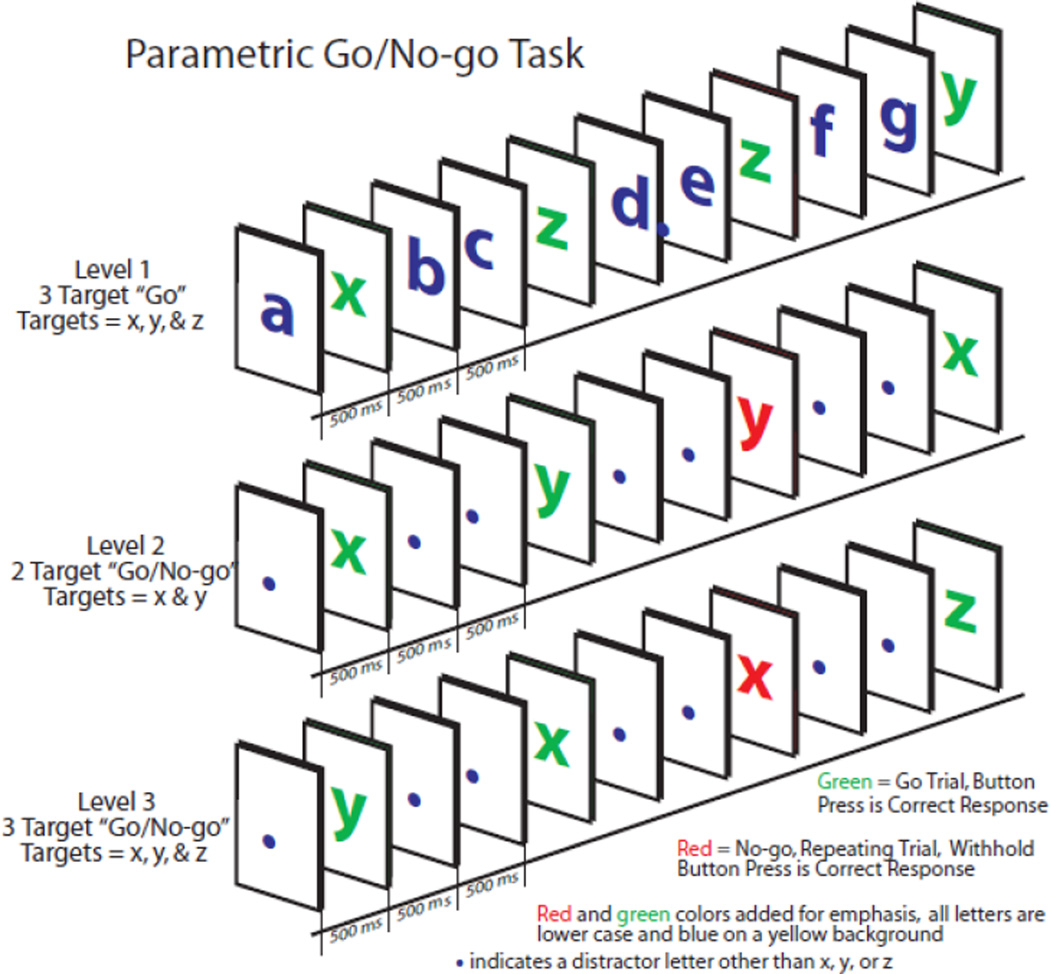
Parametric Go/No-go Task (PGNG; Langenecker et al., 2005)
The PGNG is an eleven minute task which measures attention (hits) and set-shifting, processing speed and correct (rejections) and incorrect (commissions) responses to lure trials as a part of inhibitory control. The PGNG task consists of three separate levels, which were completed in order of ascending difficulty. For all three levels, a serial stream of letters is presented (navy blue letter in 120 point Arial font on a yellow background) for 500 ms with no ISI.
Level 1 (the 3-target “Go” condition) is designed to build and sustain prepotent responding to the set of working memory (WM) target letters (“x”, “y”, and “z”, see Figure 1). The participant responds to the WM target letters each and every time they appear, regardless of order.
Figure 1.
The Go/No-go task illustration. The non-repeating rule is illustrated for Level 2 and Level 3 Go/No-go, while the Level 1 Go task requires responses to all target stimuli regardless of order.
In Level 2, (the 2-Target Go/No-go condition) participants are required to respond to the WM target letters (“x” and “y”, “z” is absent in this level) each time they appear, in alternation or non-repeating order. This “non-repeating rule” stipulates that once the participant responds to the target “x”, the WM target set is “y” and the WM lure set is “x.” After they respond to a “y”, then the set is shifted - the WM target set is “x” and the WM lure set is “y”. Level 2 requires sustained attention, inhibitory control, and set-shifting. The task is designed so that only two targets need to be tracked and the subject is instructed to start over if they become confused about the current WM target or WM lure set. The WM target load is always 1 target letter of 2 possible. Level 3 requires sustained attention, inhibitory control, and set-shifting, much as Level 2, with three targets, and switching between 2 WM targets and 1 WM lure set.
2.1.3 Procedures
Research participants were provided with a complete description of the study and informed consent was obtained. The data obtained from clinic patients was deidentified with an IRB approved waiver of informed consent. Both MDD and HC underwent a clinical interview to confirm presence or absence of psychiatric conditions. HC status was confirmed with the SCID-IV non-patient version or DIGS. For a subset of participants MDD diagnosis was confirmed with the SCID-IV patient version (n = 15) or with the DIGS (n = 8). The remaining MDD participants (n = 93) underwent diagnostic interview by board-certified psychiatrists during intake sessions at the University of Michigan’s Department of Psychiatry.
For MDD patients recruited at intake, a medical assistant greeted the participant prior to the diagnostic interview and explained the purposes of the testing. The assistant indicated that certain cognitive problems may often be found in depression and may differ based upon smoking status. The normative performance data were then provided to the diagnostic and treating clinician to assist with treatment planning, typically at the end of the visit. HC and MDD participants recruited through research studies completed the clinical interview and were then administered the neuropsychological and self-report measures, typically on the same day. For all participants and patients, the assistant assured the participant that the cognitive tasks were not designed for perfect performance, nor was the screen designed as a thorough measure of cognitive functioning. The assistant then administered the three computer-based tasks. Finally, participants completed a packet of questionnaires that included the PHQ-8 or PHQ-9, FTND, and SHQ.
Research participants were compensated with monetary payment for their participation at the end of the study visit, typically at $15 per hour. Clinical patients were not compensated, and received the clinical screening evaluation free of charge through the University of Michigan Depression Center.
2.1.4 Statistical Analyses and Covariates
As we were interested in the lifetime impact of smoking history on cognitive function, rather than the state-based characteristics, current and ex-smokers were combined into an ever-smoking group. Ever-smoking is by standard criteria of greater than 100 cigarettes lifetime (Bondy, Victor, & Diemert, 2009). The never smokers (NS) were the comparison group. A repeated measures MANCOVA was run with smoking history (ever smokers (ES) vs. NS) and diagnostic group (MDD vs HC) as the independent variables and executive measures as the dependent variables, with gender as a covariate. The executive functioning variables were attention and set-shifting, inhibitory control, inhibitory processing speed for each level of the PGNG task. An MANCOVA was computed with smoking history (ES vs NS) and diagnostic group (MDD vs HC) as the independent variables and emotional processing as the dependent variables, with gender as a covariate. The emotional processing variables were accuracy of face detection and reaction time of accurately detecting faces on the FEPT task. Posthoc analyses were run on cognitive variables with smoking-related variables. All analyses were computed with current and ex-smokers combined into the ES group. Comparisons between the current and ex-smoker groups on the dependent variables of interest were non-significant (F’s (1,47) < 3.0, p’s >.05), supporting collapsing these groups into one ES group. In addition, current and ex-smokers did not differ on IQ, age, education, or gender (% female).
The effects of medication were also analyzed, if in a limited fashion. The sample size was small, and medications were not randomly administered. We investigated the relationship between number of psychotropic medications and cognitive performance, to assess any severity by performance effects. The number of psychotropic medications was not a significant covariate for any of the cognitive variables, p-values > .15.
3.1 Results
3.1.1 Executive functioning performance
A repeated measures MANCOVA was computed with the type of PGNG measure (sustained attention, inhibitory control, and processing speed) and level of cognitive load (level 1, level 2, and level 3) as the within-subject dependent variables, and diagnostic group (MDD, HC), as well as smoking group (ES, NS) as the independent variables, controlling for gender. The Wilks Lambda multivariate test of overall differences was significant for the interaction between diagnostic group and type of PGNG measure (F(2,184) = 3.36, p = .04), but was not significant for the interaction between smoking group and type of PGNG measure (F(2,184) = 0.34, p = .72), nor for the interaction of diagnostic group, smoking group, and type of PGNG measure (F(2,184) = 0.05, p = .95). Similarly, the Wilks Lambda multivariate test of overall differences were not significant for the interaction between cognitive load and diagnostic group (F(2,184) = 1.33, p = .27) or smoking group (F(2,184) = 0.07, p = .93). Nor was there an interaction between cognitive load, diagnostic group, and smoking group, F(2,184) = 0.68, p = .51. Univariate between-subjects tests indicated that MDD performed significantly more poorly overall than HC (F(1,185) = 6.20, p = .01), but that ES and NS had non-significant differences in performance, F(1,185) = 0.03, p = .86. There was no significant interaction between diagnostic group and smoking group on overall performance, F(1,185) = 0.08, p = .77. Follow-up ANOVAs revealed that mean response time for targets on the PGNG task was significantly slower in MDD than HC (F(2,189) = 10.38, p = .002, n2 = .05), but performance did not differ between groups on inhibitory control accuracy (F(2,190) = 1.42, p = .24) or attention accuracy, F(2,190) = 2.49, p = .12.
3.1.2 Emotional processing performance
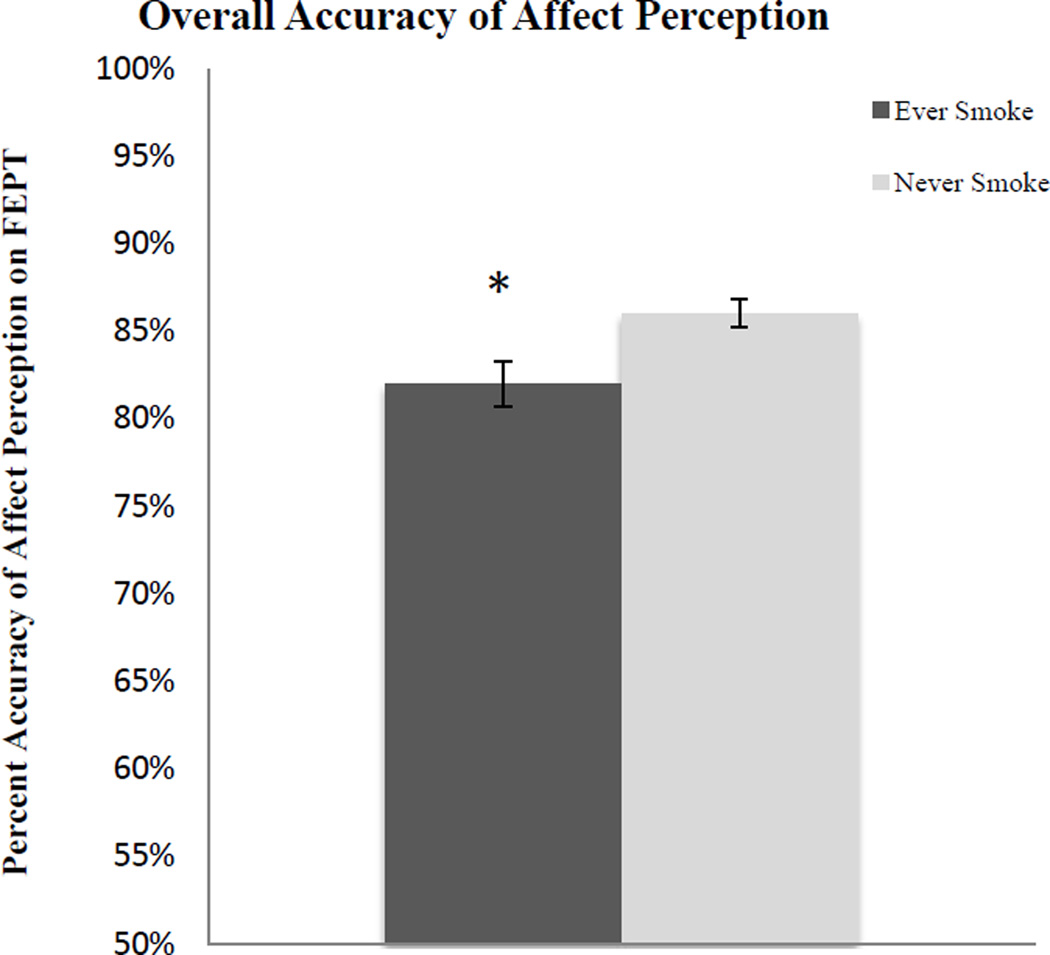
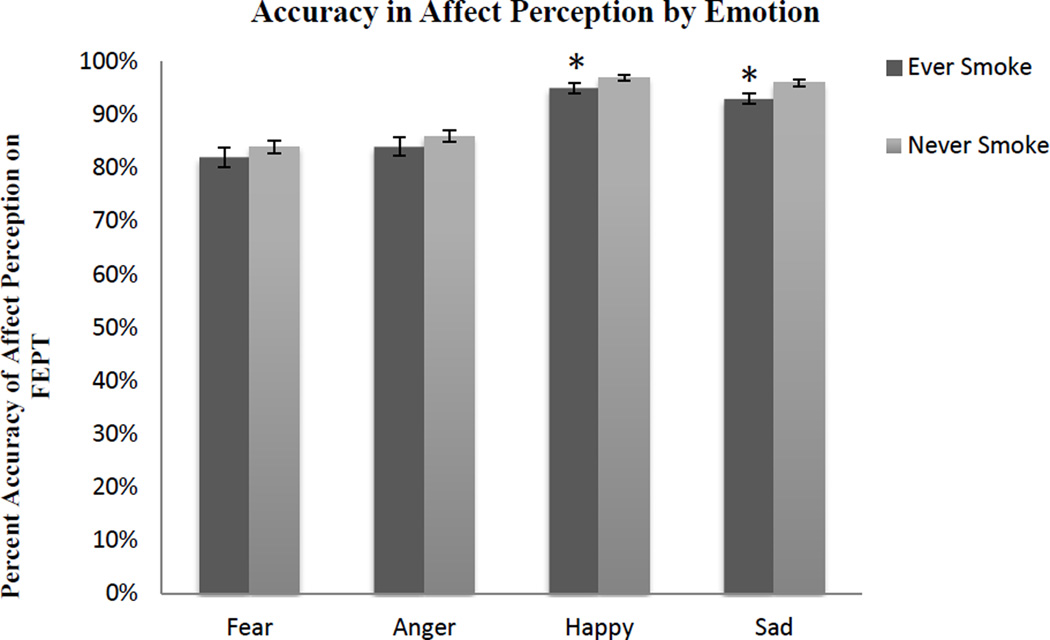
ANCOVAs were computed with face accuracy and reaction time to correct faces as separate dependent variables and diagnostic group (MDD, HC), as well as smoking group (ES, NS) as the independent variables, controlling for gender, in each analysis. Results indicated that in general, HC and MDD did not differ on accuracy of identifying emotions, F(4,186) = 0.001, p = .98. On the other hand, ES were significantly worse at identifying emotions in general than NS (see Figure 2), F(4,186) = 7.12, p < .01. Post-hoc analyses indicated that ES were significantly poorer at accurately detecting happy and sad faces (see Figure 3), but not fearful or angry faces (see Table 2).
Figure 2.
Facial Emotion Perception Test overall accuracy of affect perception. ES were significantly worse at identifying emotions than NS when controlling for gender.
Figure 3.
Facial Emotion Perception Test accuracy by emotion. ES were significantly worse at accurately detecting happy and sad faces, including controlling for gender.
Table 2.
Post-hoc ANOVAs for accuracy of affect perception by smoking group.
| Emotion | df | F | n2 | p |
|---|---|---|---|---|
| 1) Fear | 2 | 2.35 | 0.01 | .13 |
| 2) Anger | 2 | 0.57 | 0.00 | .45 |
| 3) Happy | 2 | 4.53 | 0.02 | .04 |
| 4) Sad | 2 | 4.31 | 0.02 | .04 |
There was no interaction between diagnostic group and smoking group on performance accuracy, F(4,186) = 0.16, p = .69. Further, HC and MDD did not differ on reaction time to correct faces, (F(4,166) = 0.84, p = .36), nor did NS and ES, F(4,166) = 0.43, p = .51. There was no interaction between diagnostic group and smoking group on reaction time to correct faces, F(4,166) = 0.04, p = .84.
We used a MANCOVA to examine participants’ bias in responding to neutral faces (when neutral is not a choice). The percent of neutral faces that participants identified as fearful, angry, happy, sad, other, or faces that were not responded to were entered as separate dependent variables and diagnostic group (MDD, HC), as well as smoking group (ES, NS) served as the independent variables, controlling for gender. The Wilks Lambda multivariate test of overall differences were significant for diagnostic group (F(6,161) = 6.84, p < .001) and smoking group (F(6,161) = 4.51, p < .001) and the interaction between diagnostic group and smoking group trended toward significance, F(6,161) = 2.05, p = .06. Univariate between-subjects tests indicated that MDD and HC did not differ on percent of neutral faces identified as fear, anger, happy, sad, or no response, p’s ≥ .10. On the other hand, ES had a significantly higher non-response rate to neutral faces than NS (F(1,166) = 22.88, p < .001, n2 = .12), but no other group differences were found between ES and NS, p-value’s ≥ .10.
4.1 Discussion
The present study provides evidence for distinctive cognitive and affective decrements in depression and cigarette smoking. Surprisingly, our hypotheses of shared dysfunction in MDD and smoking were not observed. MDD was associated with executive functioning decrements, with slower inhibitory processing speed in MDD irrespective of smoking status. Emotion processing was impacted by a positive smoking history. These effects did not overlap across diagnostic groups, providing no support for our hypothesis that there may be shared effects of or risk factors for illness.
The intriguing finding from this study was that emotion perception difficulties were solely linked to positive smoking history. We found that ES demonstrated decreased accuracy overall for identification of faces, specifically for happy and sad faces. This finding suggests possibly impaired socioaffective processing among individuals with a history of smoking. Consistent with previous findings, lower accuracy in identifying happy faces in ES relative to NS may suggest that smokers tend to undervalue natural rewards (Kahler, et al., 2012). Decreased accuracy in identifying sad faces, along with happy faces, may suggest disrupted hedonic processing in ES. Dinn, Aycicegi, and Harris (2004) found support for the latter hypothesis, suggesting that diminished response to reward and punishment cues might reflect underlying orbitofrontal dysfunction. It is possible that disrupted hedonic processes could predispose individuals to substance use, which may partially explain the high comorbidity between MDD and smoking. It is also possible that smoking may alter the neural and biological mechanisms, directly disrupting hedonic processing and increasing the risk of depression.
With respect to interventions aimed at smoking cessation, the findings of the present study suggest that the measures described could be used as a brief screener to identify individuals who may have emotion perception deficits. It has been suggested that escape and avoidance of negative affect has been described in the literature as a possible motive for drug use (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Emotional intelligence, in part comprised of the ability to accurately perceive emotions, may mediate the relationship between negative affectivity and nicotine dependence (Mayer, Salovey, Caruso, & Sitarenios, 2001; Carmody, Vieten, & Astin, 2007). Thus, enhancing one’s ability to effectively recognize and respond to both external and internal emotional cues could lead to greater success in smoking cessation attempts. Batra and colleagues (2010) reported that individuals with elevated depressive symptoms who underwent smoking cessation treatment with an emotion regulation component demonstrated abstinence rates that were three times greater than those with elevated depressive symptoms who received a standard cessation treatment. In addition, treatments can target deficits that may be related to smoking or depression based upon these brief screens, with the objective of strengthening weak skills that may be a risk for or a comorbid outcome of the illnesses.
It was expected that inhibitory control accuracy and inhibitory processing speed measures would be related to smoking and depression. In fact, processing speed was found to be related only to depression, not to smoking. Inhibitory control difficulties were not observed in either condition. Response times to targets in MDD were significantly slower than in HC, suggesting decreased processing speed in MDD. It is important to note that slowed reaction times without reductions in accuracy may indicate a trade-off between response time and accuracy in an effort to avoid errors of commission (Langenecker, et al., 2007). Also, it is possible that the relatively low levels of nicotine dependence in our study may reflect a less severe condition whereby response inhibition is preserved (Votubra & Langenecker, 2013), or that this EF task is not sensitive to inhibitory control difficulties of smokers.
There are some limitations to acknowledge in this study. It is retrospective, with selective recruitment based upon depression status and individuals who were arriving to a tertiary care academic medical center for treatment or to volunteer for research studies. Smoking rates were significant, but still relatively low, reflective of the generally lower smoking rate in a highly educated, high SES population. Further, a highly educated group may have a greater degree of cognitive reserve. Sample sizes were not equal, and included a relatively small HC ES group, potentially limiting our ability to detect interactions between MDD and ES. Finally, the Fagerstrom scores in the sample indicate a relatively lower rate of dependence than what might be observed in most studies of smoking.
In conclusion, this is an initial study investigating affective and executive function processes in individuals with and without depression and a history of smoking cigarettes. The results provide preliminary evidence for distinctive cognitive decrements in smokers and individuals with depression. The study provides a novel set of hypotheses to probe in future studies of comorbidity examining distinct and shared mechanisms of risk. Prospective, controlled studies with a more representative sample of this special population are needed, and should include recruitment of current, former, and never smokers both with and without a history of MDD.
Highlights.
We examine the impact of positive smoking history and major depressive disorder (MDD) on emotion perception and executive functioning.
Positive history of smoking was found to be associated with reduced emotion perception accuracy.
MDD was associated with poorer executive functioning performance.
Additive decrements in individuals with both a history of smoking and MDD were not observed.
Acknowledgments
Role of Funding Sources
Funding for this study was provided by the Rachel Upjohn Clinical Scholars Award (to SAL), K-23 grant MH074459 (SAL), NIH grant P01MH 42251 (to EAY, JKZ), T32MH067631 (to NAC) from the National Institute on Mental Health (NIMH),internal support from the Depression and Neuropsychology Sections of the Department of Psychiatry, University of Michigan Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Contributors
KKM – Writing, literature search, review
NAC – Writing, statistics, literature search, review
RO – Writing, statistics, literature search
JKZ – Writing, planning, review
BG - Writing, design, planning, review
CSP - Writing, design, planning, review
JCH - Writing, planning, review
SAL - Writing, design, statistics, planning, review
Works Cited
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Batra A, Collins SE, Schroter M, Eck S, Torchalla I, Buchkremer G. A cluster-randomized effectiveness trial of smoking cessation modified for at-risk smoker subgroups. Journal of Substance Abuse Treatment. 2010;38:128–140. doi: 10.1016/j.jsat.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tobacco Control. 2009;18(4):317–323. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Gordijn MC. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: A longitudinal study. The Journal of Nervous and Mental Disease. 1999;187(10):595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: A review. Australian and New Zealand Journal of Psychiatry. 2010;44:681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180–185. [PubMed] [Google Scholar]
- Carmody TP, Vieten C, Astin JA. Negative affect, emotional acceptance, and smoking cessation. Journal of Psychoactive Drugs. 2007;39(4):499–508. doi: 10.1080/02791072.2007.10399889. [DOI] [PubMed] [Google Scholar]
- Carton JS, Kessler EA, Pape CL. Non-verbal decoding and relationship wellbeing in adults. Journal of Nonverbal Behavior. 1999;23:91–100. [Google Scholar]
- Charbol H, Niezborala M, Chastan E, de Leon J. Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addictive Behaviors. 2005;30(7):1474–1477. doi: 10.1016/j.addbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990;31(4):350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Stolar M, Merikangas KR. Smoking and depression: An examination of mechanisms of comorbidity. American Journal of Psychiatry. 2002;159:947–953. doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Aycicegi A, Harris CL. Cigarette smoking in a student sample: Neurocognitive and clinical correlates. Addictive Behaviors. 2004;29:107–126. doi: 10.1016/j.addbeh.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: Implications for neurocognition and brain neurobiology. International Journal of Environmental Research and Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25(3):313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. SCID-I/P Version 2.0). [Google Scholar]
- Gilbert DG, Gilbert BO. Personality, psychopathology, and nicotine response as mediators of the genetics of smoking. Behavior Genetics. 1995;25(2):133–147. doi: 10.1007/BF02196923. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, Smoking Cessation, and Major Depression. Journal of the American Medical Association. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Gollan JK, McCloskey M, Hoxha D, Coccaro EF. How do depressed and healthy adults interpret nuanced facial expressions? Journal of Abnormal Psychology. 2010;119(4):804–810. doi: 10.1037/a0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Gur R, Edwin R, Gur R, Zwil A, Heimberg C, Kraemer H. Facial emotion discrimination II: Behavioral findings in depression. Psychiatry Research. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hale WW. Judgment of facial expressions and depression persistence. Psychiatry Research. 1998;80:265–274. doi: 10.1016/s0165-1781(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. Journal of General Internal Medicine. 2006;21(6):547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Brooks BL, Langenecker SA, Young AH. Identifying a cognitive impairment subgroup in adults with mood disorders. Journal of Affective Disorders. 2011;132:360–367. doi: 10.1016/j.jad.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WF, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Kahler CW, McHugh RK, Leventhal AM, Colby SM, Gwaltney CJ, Monti PM. High hostility among smokers predicts slower recognition of positive facial emotion. Personality and Individual Differences. 2012;52:444–448. doi: 10.1016/j.paid.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. American Journal of Epidemiology. 2002;156(10):936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- Kampf-Sherf O, Zlotogorski Z, Gilboa A, Speedie L, Lereya J, Rosca P, Shavit Y. Neuropsychological functioning in major depression and responsiveness to selective serotonin reuptake inhibitors antidepressants. Journal of Affective Disorders. 2004;82:453–459. doi: 10.1016/j.jad.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: A quantitative review. Psychiatry Research. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):509–515. [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta J-K, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27(3):320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Caveney AF, Giordani B, Young EA, Nielson KA, Rapport LJ, Zubieta J-K. The sensitivity and psychometric properties of a brief computer-based screening battery in a depression clinic. Psychiatry Research. 2007;152:143–154. doi: 10.1016/j.psychres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Finkenauer R, Snedecor SM, Zubieta J-K, Young EA, Marcus SM, Pomerleau CS. Sex differences in smoking prevalence and characteristics associated with receptivity to quitting in psychiatric patients. In: Hernandez P, Alonso S, editors. Women and Depression. New York: Nova Science Publishers; 2009. pp. 1–14. [Google Scholar]
- Langenecker SA, Lee HJ, Bieliauskas LA. Neuropsychology of depression and related mood disorders. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. 2009:523–559. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual. Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Murray CJ. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Emotional intelligence as a standard intelligence. Emotion. 2001;1(3):232–242. [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Actual causes of death in the United States. The Journal of the American Medical Association. 1993;270(18):2207–2212. [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, London ED. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. Addictive Behaviors. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Reich T. Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Archives of general psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 89:125–135. doi: 10.1016/j.jad.2005.09.006. (20005). [DOI] [PubMed] [Google Scholar]
- Porter RJ, Bourke C, Gallagher P. Neuropsychological impairment in major depression: Its nature, origin, and clinical significance. Australian and New Zealand Journal of Psychiatry. 2007;41:115–128. doi: 10.1080/00048670601109881. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. Depression and smoking in the US household population aged 20 and over, 2005–2008. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- Rapport LJ, Friedman SL, Tzelepis A, VanVoorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16(1):102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: Evidence from a prospective birth cohort study. Research and Practice. 2003;93(6):994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Kato N. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of living scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- Spada MM, Nikcevic AV, Moneta GB, Wells A. Metacognition as a mediator of the relationship between emotion and smoking dependence. Addictive Behaviors. 2007;32:2120–2129. doi: 10.1016/j.addbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Suchanek Hudmon K, Pomerleau CS, Brigham J, Javitz H, Swan GE. Validity of retrospective assessments of nicotine dependence: A preliminary report. Addictive Behaviors. 2005;30(3):613–617. doi: 10.1016/j.addbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology Review. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Trosclair A, Dube SR. Smoking among adults reporting lifetime depression, anxiety, anxiety with depression, and major depressive episode, United States, 2005–2006. Addictive Behaviors. 2010;35:438–443. doi: 10.1016/j.addbeh.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Tsoh JY, Sharon MH. Depression and smoking: From the Transtheoretical Model of change perspective. Addictive Behaviors. 2004;29:801–805. doi: 10.1016/j.addbeh.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Francis T, Minor K, Thomas A, Stone WS. Genetics of smoking and depression. Human Genetics. 2012;131:905–915. doi: 10.1007/s00439-012-1170-6. [DOI] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addiction Biology. 2010;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votubra KL, Langenecker SA. Factor structure, construct validity, and age- and education-based normative data for the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2013;35(2):132–146. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Reutenauer EL, Allen TM, Termine A, Vessicchio JC, Sacco KA, George TP. Reliability of the Fagerstrom Test for Nicotine Dependence, Minnesota Nicotine withdrawal Scale, and Tiffany questionnaire for Smoking Urges in smokers with and without schizophrenia. Drug and Alcohol Dependence. 2007;86(2–3):278–282. doi: 10.1016/j.drugalcdep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wright SL, Langenecker SA, Deldin PJ, Rapport LJ, Nielson KA, Kade AM, Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosomatic Medicine. 1999;61(1):6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- Zubieta J-K. Gender-specific disruptions in emotion processing in younger adults with depression. Depression and Anxiety. 2009;26(2):182–189. doi: 10.1002/da.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]