Abstract
Optical coherence tomography (OCT) has become essential to evaluate axonal/neuronal integrity, to assess disease progression in the afferent visual pathway and to predict visual recovery after surgery in compressive optic neuropathies. Besides that OCT testing is considered a powerful biomarker of neurodegeneration and a promising outcome measure for neuroprotective trials in multiple sclerosis (MS).
Currently, spectral-domain OCT (SD-OCT) technology allows quantification of retinal individual layers. The Ganglion Cell layer (GCL) investigation has become one of the most useful tools from a neuro-ophthalmic perspective. It has a high correlation with perimetry, is predictive of future progression and is a highly sensitive, specific of several neuro-ophthalmic pathologies. Moreover the superior correlation with clinical measures compared to peripapillary retinal nerve fiber layer (pRNFL) suggests that GCL analysis might be a better approach to examine MS neurodegeneration.
In disorders with optic disk edema, such as ischemic optic neuropathy, papillitis and papilledema, reduction in RNFL thickness caused by axonal atrophy is difficult to distinguish from a swelling resolution. In this setting, and in buried optic nerve head drusen (ONHD), GCL analysis may provide more accurate information than RNFL analysis and it might be an early structural indicator of irreversible neuronal loss.
Enhanced depth imaging OCT (EDI-OCT) provides in vivo detail of ONHD, allowing to evaluate and quantify the drusen dimensions.
OCT is improving our knowledge in hereditary optic neuropathies. Furthermore, there is growing evidence about the role of OCT as an adjunctive biomarker of disorders such as Alzheimer and Parkinson’s disease.
Keywords: Neuro-ophthalmology, Optical coherence tomography, Ganglion cell layer
Introduction
Optical coherence tomography (OCT) has revolutionized ophthalmology and it has become one of the most important tools in neuro-ophthalmic practice.
Time-domain OCT (TD-OCT) was widely used in clinical practice and currently replaced by the newer Spectral-Domain OCT (SD-OCT) technology that offers considerable improvements. SD-OCT has faster acquisition time and higher resolution than TD-OCT, providing high quality three dimensional images. Furthermore it can track eye movements and it has eliminated operator bias with automatic centering.
OCT is a quick, sensitive, non-invasive, user-friendly device that provides high-resolution images of the peripapillary retinal nerve fiber layer (pRNFL), macular volume, macular ganglion cell layer (GCL), and optic nerve head, yielding reproducible and reliable measurements. OCT allows us to search about axonal-neuronal integrity in the afferent visual pathway and, compared with perimetry, is faster, more reproducible, precise and less dependent on patient.
Depending on the type of disorder, OCT provides data relevant for diagnosis, follow-up, and prognosis. Although diagnosis exclusively based on OCT is not possible, in some diseases there are pathognomonic findings leading to correct diagnosis. This review gives an overview on current applications, typical changes, new perspectives and future directions of the OCT in the following diseases: optic neuritis (isolated or associated with multiple sclerosis or neuromyelitis optica), anterior ischemic optic neuropathy, papilledema, optic nerve head drusen, autosomal dominant optic atrophy, Leber hereditary optic neuropathy and neurodegenerative diseases.
Optic neuritis/multiple sclerosis
Multiple sclerosis (MS) is a disorder characterized by inflammation and neuro-axonal degeneration. Optic neuritis (ON) is one of the manifestations of MS and is the presenting event in 25–50% of MS cases. Given its high degree of reliability, sensitivity and ease of use, OCT is an ideal method for assessing pathologic changes in the anterior visual pathway of patients with ON and MS.
Retinal nerve fiber layer thickness
Firstly reported by Parisi in 1999, thinning of pRNFL by OCT, is a well-documented structural marker of axonal degeneration in MS, which occurs even in the absence of optic neuritis (ON).1–6
OCT confirms the presence of optic disk edema in anterior ON, and quantifies the severity of axonal loss that follows the acute episode. OCT can detect subclinical axonal loss in eyes with normal visual fields and normal visual acuity (Figs. 1 and 3).7
Figure 1.
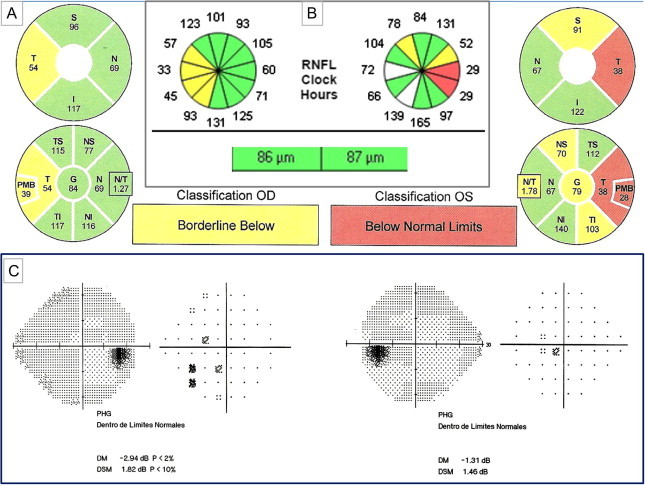
A 32 year-old woman diagnosed of remitting-relapsing multiple sclerosis (RRMS) with ON antecedent just in the left eye having bilateral RNFL thinning by Spectralis (A) and Cirrus-OCT(B) although more accentuated in the involved left eye. Damage predominates in the temporal quadrant in both eyes. Despite RNFL thinning, visual acuity is 20/20 in both eyes and visual fields remain normal (C).
Figure 3.
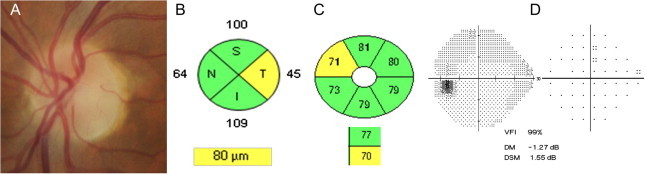
Axonal and neuronal damage at 6 months after a left anterior optic neuritis. Temporal optic disk pallor (A), temporal RNFL thinning (B) and the corresponding supero-nasal CGL thinning (C). Visual field is normal (D).
Meta-analyses of data for TD-OCT show that RNFL thinning is milder in MS without ON (7.08 μm) than in eyes that have suffered ON (20.38 μm) and it is more pronounced in the temporal quadrant.4
Rebolleda et al. demonstrated by OCT an enlarged cup to disk ratio after unilateral ON that correlated with RNFL thinning (Fig. 2). A cupping asymmetry equal or greater than 0.2 was present in around 25% of cases.8
Figure 2.
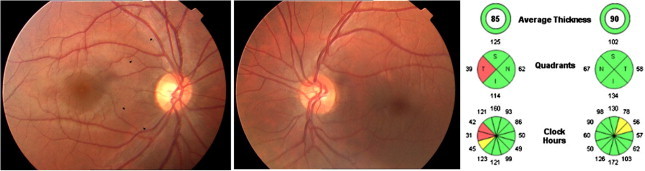
Acquired optic disk cupping after an optic neuritis episode in the right eye. Temporal RNFL loss is shown in fundus photograph (black arrows). RNFL thinning predominates in the temporal quadrant (OCT-Cirrus).
RNFL thickness correlates with visual and neurological functioning as well as with paraclinical data and disease duration. The strongest correlations were shown in studies including patients with MS and ON antecedent.3,4,9
A moderate association has also been shown between the RNFL thickness and several magnetic resonance imaging (MRI) findings characteristic of brain atrophy.3,10 A strong correlation with MRI results is unlikely since axons are not the only component of the brain and brain atrophy also reflects loss of myelin, gliosis, synaptic and water content changes.
Studies using newer SD-OCT corroborate previous findings by TD-OCT, where RNFL thinning typically occurs in the temporal quadrant,5,11–13 although measurement values cannot be directly compared and there is also a substantial color-code disagreement among SD-OCT devices (Fig. 1).13,14
Spectralis-OCT incorporated the N-site axonal protocol which differs from the standard pRNFL scan because it starts and terminates in the nasal side of the optic nerve, focusing on the temporal quadrant. Using this protocol in patients with remitting-relapsing MS (RRMS), Spectralis yields a significantly higher thinning for the temporal quadrant than OCT Cirrus in non-ON eyes, suggesting that N-site axonal analysis could define axonal damage earlier than conventional RNFL analysis.5
Macular and Ganglion cell layer thickness
Initially, OCT focused primarily on the evaluation of pRNFL. The latest OCT investigations involve segmentation of specific retinal layers allowing quantification of both axonal damage (RNFL) and neuronal degeneration. Several studies have shown that patients with RRMS exhibit a significant thinning of both, pRNFL and ganglion cell layer (GCL),15–19 and the damage is more pronounced in eyes with ON antecedent (Fig. 1).
OCT-GCL thickness analysis is more sensitive to detect damage and is reduced before than pRNFL analysis in RRMS patients, even in the absence of a previous episode of ON.18,19 Unsurprisingly, the sectors showing the highest abnormality rate in GCL analysis are the supero and infero-nasal (Fig. 3).20 Moreover, macular GCL thickness correlates better with visual dysfunction [visual acuity (VA), low-contrast letter acuity, and vision-specific quality of life measures, visual field mean deviation (MD)], disability and MRI than RNFL thickness.15,18
The improved reproducibility of SD-OCT enhances the value of the analyzed data from individual quadrants in the longitudinal evaluation of MS patients.21,22 Progressive RNFL and ganglion cell inner plexiform layer (GCIPL) thinning occurs as a function of time in patients with MS, even in the absence of ON, and is associated with clinically significant visual loss.23–27 Narayanan reported that RNFL and GCIPL decreased with follow-up time by −1.49 and −0.53 μm/year, respectively.25 Progressive changes seem to correlate with changes in neurological impairment measured by the Expanded Disability Status Scale (EDSS).
Macular edema and MS
SD-OCT has become the most useful tool for the diagnosis of macular edema that could appear in MS patients with intermediate uveitis or as a side effect of Fingolimod (FTY-720). A review of clinical trials reported that Fingolimod 0.5 mg and 1.25 mg were associated with a low incidence of macular edema (0.3% and 1.2%, respectively) that is a dose-dependent adverse event and typically resolves upon cessation of therapy. An ophthalmic examination before initiating fingolimod therapy and regular follow-up eye examinations by OCT during therapy are recommended.28
In 2012, Gelfand et al.29 identified by OCT a microcystic macular edema (MME) in patients with demyelinating optic neuropathy. MME is characterized by small hyporeflective spaces within the inner nuclear layer (INL) in the parafoveal region of the retina. In microvacuolar aspect, the sparing of fovea and the limitation to the INL distinguish MME from other macular edemas caused by vascular leakage (Fig. 4).
Figure 4.
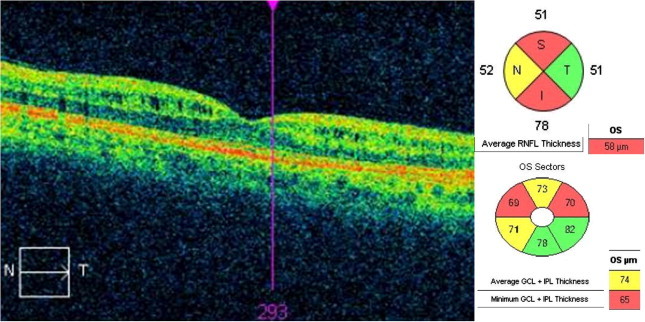
Patient with RRMS, showing in the left eye a microcystic macular edema located in the inner nuclear layer, nasal and temporally. There is a significant thinning of both RNFL and GCIPL thickness (image provided by Santos-Bueso E).
MME and thickening of the INL on OCT were associated with disease activity and worse disability in MS, leading to consider as useful predictor of disease progression in patients with MS.30 However, this finding is not specific for demyelinating disease and can be found in numerous disorders such as compressive, nutritional/toxic, and hereditary optic neuropathies. Nowadays, MME is considered a sign of optic neuropathy irrespective of its etiology and the distinctive intraretinal anatomy suggests that it is caused by retrograde degeneration of the inner retinal layers, resulting in impaired fluid resorption in the macula.31
MS subtypes and neuromyelitis optica
OCT measurements differ among MS subtypes.32 RNFL thinning in the secondary progressive MS (SPMS) forms is more pronounced than in patients with RRMS, and total macular volumes are lower in SPMS than in primary progressive MS (PPMS). Saidha et al. reported thinning of the GCIPL in all MS subtypes but it was most prominent in SPMS.18
Several reports have reported that visual prognosis is much worse and axonal loss is greater after an episode of ON in patients with neuromyelitis optica (NMO) than in patients with MS, even after adjusting the RNFL for visual outcome. It has also been shown that the superior and inferior quadrants are more intensely affected after NMO.33–36
Moreover, patients with NMO and longitudinally extensive transverse myelitis (LETM) had a significantly thicker INL than patients with MS.36,37
Although a critical value of RNFL thickness has been proposed, there was a considerable overlap in OCT measurements between MS and NMO, limiting the role of OCT to differentiate the two conditions on an individual basis.
Prognostic value and future developments of OCT in MS
In addition to evaluate the damage (axonal and neuronal loss) after each episode of ON and monitor the changes, OCT may predict visual recovery.
Costello et al. found a threshold of RNFL thickness (75 μm) below which RNFL measurements predicted persistent visual dysfunction with TD-OCT.23,24 More recently, they confirm these findings using SD-OCT technology. Both one-month RNFL and GCIPL values after ON predicted change in visual field MD and VA at 6 and 12 months.38
In anterior optic neuritis, RNFL measurements obtained closer to the acute episode can underestimate the amount of true damage due to the swelling could mask the true degree of RNFL loss (Fig. 5). Contrary to RNFL evaluation, macular thickness and GCL changes are not influenced by the optic nerve edema and could be more useful for detecting the structural changes in the first month.39
Figure 5.
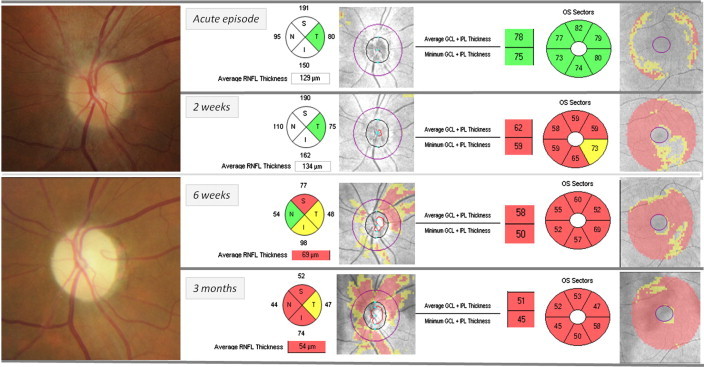
Left anterior optic neuritis. Ganglion cell layer thinning occurred as early as at 2 weeks after the acute episode, when optic disk swelling masked the RNFL damage. One month later, once edema has resolved, both axonal and neuronal damage can be quantified. Hence, the presence of GCL damage during optic disk edema has a striking prognostic value.
Kupersmith observed in eyes suffering anterior ON, that at 1 month only 10% had RNFL loss, while near 70% of eyes had GCL + IPL thinning.40 According to these data and in author’s experience, CGL thinning can develop rapidly after ON, and therefore it might be considered a biomarker of early structural loss.
The study of individual retinal layers opens up new perspectives in MS. Saidha et al. identified a subset of MS patients with a unique pattern of retinal neuronal cell loss (predominant macular thinning phenotype) in whom there appears to be disproportionate thinning of the inner and outer nuclear layers with relative sparing of the GCL and more rapid disability progression.41 These findings support the possibility of primary retinal pathology may be a harbinger of a more aggressive form of MS.
OCT has been included in the most recent MS clinical trials, and now is considered essential for assessing non-invasively effectiveness of therapies that reduce axonal and neuronal loss by neuroprotective or myelin repair mechanisms. A combination of RNFL plus individual layers in the macula may give the most complete picture of disease activity in MS and ON, for study and therapeutic purposes focused on axonal loss, neuronal degeneration or both. Moreover, clinical trials have benefited from the presence of OCT reading centers where certified technicians and prompt transmission of data for ongoing quality control monitoring provide higher data quality.
Developments that could improve understanding of MS in the future include OCT angiography, polarization-sensitive OCT (PS-OCT), and fluorescence labeling.4
There is some evidence that vascular co-morbidity is a poor prognostic sign in MS. Swept-Source OCT angiography that provides flow index measurements could be suitable for evaluating optic nerve head and parafoveal perfusion in MS patients.4,42
PS-OCT yields information about any light polarization changing related to tissue birefringence. The birefringence of the RNFL is related to the structure of neurofilaments and microtubules and is not constant. Because of changes the axonal cytoskeleton can precede axonal loss, PS-OCT might be an opportunity to detect early stages of axonal pathology in MS.43
Ischemic optic neuropathy
As in anterior ON, OCT measures optic disk edema and monitors RNFL loss over time in patients with anterior ischemic optic neuropathy (AION).
Morphological changes comprise severe swelling after onset of AION, which rapidly turns into atrophy. Contreras et al. reported that mean OCT-measured RNFL thickness increased to 96.4% in the affected eye compared with the fellow eye at the onset of nonarteritic AION (NAION). Already after 2 months, more than 80% of the patients showed a RNFL thinning. Progressive RNFL thinning between month 2 and month 4 suggests ongoing atrophy, whereas a stable morphologic end point is reached after month 6.44–46
In the acute phase of AION, optic disk and axonal swelling prevents by RNFL thickening to detect axonal damage. As previously commented in anterior ON, a useful alternative approach is to analyze GCL. Kupersmith demonstrated that at 1 month, only 10% eyes with NAION had RNFL loss, while 76% had GCIPL thinning.40 Therefore GCL thinning can be detected prior to RNFL loss, and could be a biomarker of early structural loss in AION (Fig. 6).
Figure 6.
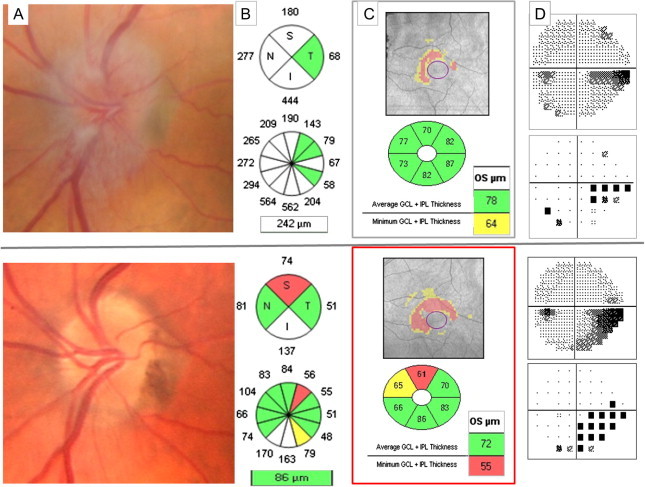
Left anterior ischemic optic neuropathy. Top row: One week after acute visual acuity loss. (A) Optic nerve swelling causes RNFL thickening that prevent evaluating if there is or not associated axonal loss (B). However GCIPL analysis (C) shows an abnormal thinning that correlates with the inferior VF defect (D). Bottom row: two months after the acute episode. Superior optic disk pallor (A), superior RNFL quadrant thinning (B) and GCIPL thinning (C) with the corresponding inferior VF defect (D).
A cupping enlargement is a well-known characteristic of AION caused by giant cell arteritis. Although milder, OCT has also demonstrated an enlargement of cupping after NAION (around 50% of eyes had a cup to disk ratio that differed from that in the fellow eye by more than 0.1).47,48
It has been hypothesized that most of the VA loss after a NAION episode depends on the severity of the damage to the papillo-macular bundle (PMB).49 In fact, in eyes with significantly lower VA, the RNFL thickness of the temporal quadrant by Stratus-OCT was almost 40% lower than that of the fellow eye. Moreover there is a significant correlation between nasal macular thickness by Stratus-OCT and VA in patients with AION.
Rebolleda et al. have found a significant correlation between VA and both temporal and PMB RNFL thicknesses, as well as with several GCL and macular measurements using the newer SD-OCT (ongoing study).
OCT can identify different patterns of RNFL involvement specific to different classic visual field (VF) defects in eyes with NAION. Bellusci et al. reported that eyes with VF defect confined to the inferior hemifield had RNFL involvement limited to the temporal, superior and nasal optic disk quadrants. Diffuse RNFL damage involving all quadrants around the disk was observed in eyes with diffuse VF loss and eyes with central or centro-cecal scotoma revealed RNFL atrophy limited to the superior and temporal sectors of the disk.51
Macula and GCL are also thinner in NAION eyes and show stronger correlation with VF than RNFL parameters.45,52,53 With newer automated segmentation software thinning of individual macula layers including RNFL, inner plexiform and GCL can be detected (ongoing study).
Macular analysis by OCT can demonstrate subretinal fluid in around 12% patients with NAION that may contribute to some of the visual loss associated with this condition and could account for some of the visual improvement that can follow after its resolution (Fig. 7).54
Figure 7.
Diffuse pRNFL thickening and macular subretinal fluid connected to peripapillary edema is showed by Cirrus-OCT during an acute episode of AION (A) that resolves 3 months later, leading to RNFL thickness loss in the superior and inferior quadrants (B).
Controversy exists regarding the optic disk size in NAION. Although subjects suffering NAION have lower cup to disk ratios than does the normal population, nerve fiber crowding does not mean necessarily a small optic disk. In fact, Contreras et al. did not show any significant difference in optic disk size between patients with NAION and control subjects.47 New Bruch’s membrane opening (BMO) disk size analysis could be useful in the future to assist in addressing this unanswered question.
OCT technology is a reliable and objective tool to assess new therapies arising in the future for this devastating disorder.55
Papilledema
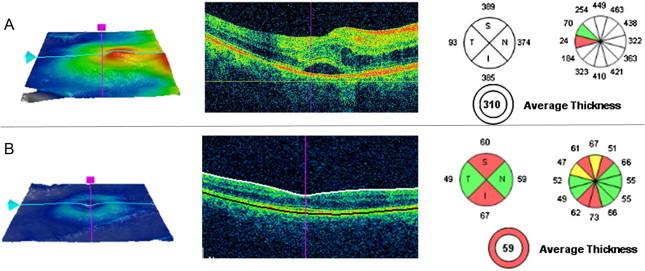
OCT provides reliable and quantitative information on optic disk edema and structural changes during the resolution of optic nerve head swelling. For lower grades of papilledema, pRNFL analysis is very useful as an adjunct method to confirm and quantify the severity of disk swelling. However, in moderate to severe papilledema (Frisén grade 3 or above), substantial thickening of the pRNFL (average RNFL >200 μm) causes the software algorithm to fail in over a third of the cases, yielding inaccurate values of RNFL thickness.56,57
A significant improvement in the quantification of papilledema was realized by segmenting the total retinal thickness (TRT) in the same peripapillary scan, since the inner and outer borders of the retina are more readily defined by automated software in the presence of moderate to severe papilledema.
Newer automated software segmentation of the retinal layers using a 3D graph-based approach has significantly improved upon the accuracy of defining the thickness of the retinal layers in papilledema, resulting in much fewer algorithm failures (Fig. 8). This approach has been used to segment the volume of the optic disk, which highly correlates with the RNFL, TRT and Frisén grade of papilledema in patients with raised intracranial pressure.58
Figure 8.
Optic disk swelling due to idiopathic intracranial hypertension Frisén stage grade 4. SD-OCT shows a significant pRNFL thickening and provides 3D-images of ONH volume and height.
All OCT parameters of optic nerve head swelling, such as average pRFNL thickness, pTRT, and optic nerve head (ONH) volume and height are correlated. Although the ONH volume correlates the best with the grading of photos, it has a modest correlation with cerebral spinal fluid opening pressure.57
Perimetric sensitivity losses tended to be greater in eyes with more edema.56
Another purpose of OCT is to differentiate if visual loss associated to papilledema is due to optic neuropathy or maculopathy to guide management decisions. If it is caused by optic neuropathy, it requires more aggressive approach than if it is due to a more benign, reversible macular abnormality such as subfoveal fluid or choroidal folds. The main problem using pRNFL analysis in the follow-up, is there is no way, based on OCT alone, to determine when edema reduces and RNFL thickness returns toward normal values, whether it is due to clinical improvement or if it is due to axon loss. Sometimes, when optic disk edema resolves, axonal damage has already occurred but yet cannot be detected anatomically, explaining why RNFL measures do not always correlate with VF loss.
The GCIPL analysis by OCT in the setting of papilledema may be suitable for the early detection of neuron loss and to identify patients needing more aggressive treatment. The hypothesis is that thinning of the GCL-IPL reveals early signs of progressive optic neuropathy in the presence of papilledema.
In the NORDIC Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), that represents the largest prospectively analyzed cohort of untreated patients with IIH, 7.3% of eyes had macular GCL + IPL thinning at presentation.59 These eyes had worse VA compared with eyes with GCL thickness equal to or greater than normal, indicating structural neuronal loss not apparent from RNFL measurements due to swelling. Larger studies will confirm whether GCL thinning at baseline is a risk factor for not improving or having a worse outcome. In authoŕs experience, a normal GCL thickness during the follow-up is a good prognosis signal for recovering (Fig. 9).
Figure 9.
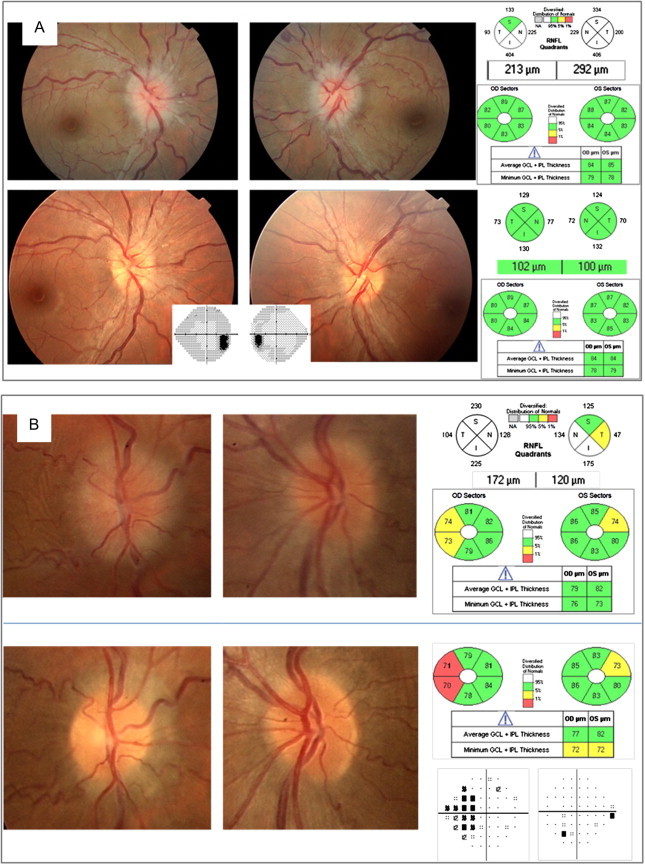
GCL-IPL analysis provides information about optic neuropathy in the presence of papilledema. (A) Idiopathic intracranial hypertension (IIH) with normal GCIPL thickness despite a significant RNFL thickening at presentation (top row). Full recovery after conservative treatment (bottom row). (B) IIH with abnormal GCIPL thinning at presentation (top row). Resolution of edema occurred after lumbo-peritoneal derivation (bottom row) surgery but RNFL, GCIPL and VF damage are present and are more severe in the eye with more edema at baseline (right eye).
In NORDIC IIHTT, GCIPL thickening occurred in 10% of eyes that was associated with thicker ONH volume, RNFL and TRT, suggesting that the axonal stasis associated to papilledema can also affect the axons immediately connected to the retina ganglion cells.59
In a minority of eyes, pRNFL and macular thickness may be subnormal despite optic disk swelling, implying a combination of swollen and thin or missing axons.
Other macular abnormalities have been discovered thanks to OCT. Photoreceptor loss has been described by SD-OCT and is associated with central/paracentral scotomas.60
Newer generation of OCT instruments with enhanced depth penetration and longer wavelength light provide greater resolution of deeper structures, such as Bruch’s membrane. Peripapillary deformations of Bruch’s membrane surrounding the neural canal due to a differential pressure between the retrobulbar optic nerve and vitreous cavity may be useful in the diagnosis and management of intracranial hypertension (IHT). In papilledema the shape of peripapillary retinal pigment epithelium (p-RPE)/Bruch membrane has an inverted U-shape compared to normals who exhibit a V-shape pointing away from the vitreous. These changes in the p-RPE-shape are dynamic and could reflect a decrease in the intracranial pressure after lumbar puncture and treatment.61,62
Another recent development in OCT that might help to understand the pathogenesis of visual loss in papilledema due to ischemia is phase contrast OCT. This technology allows to visualize capillaries and to quantify flow within a capillary bed.63
Optic nerve head drusen
Both RNFL and macular GCIPL analysis reveal significant thinning in eyes with ONHD. As drusen develop and become superficial, the RNFL thickness decreases. There is an association between OCT measurements, numbers of clinically visible ONHD and VF defects.64–66
The temporal quadrant is often undamaged, probably reflecting the preservation of central visual acuity.
Deep and buried drusen often produce a RNFL thickening that can mask the axonal damage, yielding a false negative result. In fact, Rebolleda et al. found that in eyes with buried ONHD the abnormality rate was significantly higher with GCIPL compared to RNFL evaluation. In fact, 26% of the eyes with buried ONHD, had abnormal GCIPL exams but normal RNFL thicknesses by Cirrus-OCT.67
Although further work is needed to properly establish the role of GCIPL analysis in eyes with buried ONHD, it seems that GCIPL analysis may be more useful for detecting early damage in eyes with buried drusen than RNFL exam. Longer follow-up will determine if those cases with abnormal GCIPL examination will eventually develop RNFL thinning and VF defects (Fig. 10).
Figure 10.
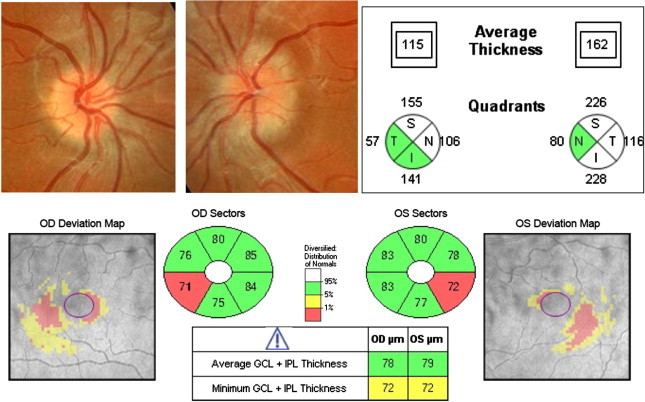
Pseudo-papilledema due to bilateral buried ONHD. A significant thickening is showing in RNFL analysis by OCT-Cirrus, preventing to detect axonal loss. GCIPL analysis is a valuable tool in this setting demonstrating an abnormal thinning in both eyes.
When ONHD is located superficially, the diagnosis is relatively easy and can be made with careful ophthalmoscopic observation. However, distinguishing papilledema from pseudopapilledema due to deep and buried drusen, can be challenging and clinical observation may be equivocal, particularly when the degree of edema is not severe (i.e. Frisén grade 1 or 2).
Several qualitative and quantitative OCT criteria have been proposed to help differentiate both disorders. The quantitative parameters have showed greater sensitivity and specificity than the qualitative criteria.68,69
Qualitative criteria for papilledema include an elevated optic nerve head with smooth internal contour and subretinal hyporeflective space (SHYPS) with recumbent “lazy V” pattern. However, ONHD displayed a “lumpy-bumpy” internal optic nerve contour and a rapid decline in the SHYPS thickness.
Quantitative comparisons include nasal RNFL and SHYPS thickness. Recently, Kulkarni et al. found no difference in RNFL thickness between buried ONHD and papilledema in any of the 4 quadrants.70
Using SD-OCT, Lee et al. reported that ONHD is visualized as a focal, hyperreflective, subretinal mass with a discrete margin.71 The outer nuclear layer smoothly covered the drusen, which led to a hyporeflective, boot-shaped area adjacent to the drusen (Fig. 11).
Figure 11.
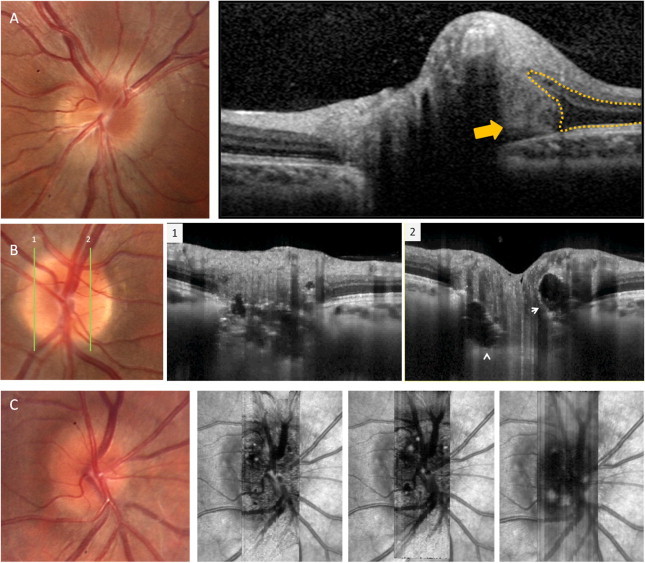
Buried optic nerve head drusen. (A) SD-OCT demonstrated the optic drusen as a focal mass with a reflectance similar to the plexiform layers (yellow arrow). The outer plexiform layer and the retinal pigment epithelium diverge as they approach the drusen given a boot-shape area adjacent to the drusen (yellow dotted line). (B) EDI-OCT along two vertical scans showing the typical ovoid areas of lower reflectivity (white arrows) bordered by hyperreflective material and multiple hyperreflective bands. C. En-face imaging that allows to visualize 3D data in a fundus projection, showing several optic nerve hyperreflective drusen along the layers of increasing depth.
Lately, several studies have assessed the value of enhanced depth imaging optical coherence tomography (EDI-OCT) in diagnosing and evaluating ONHD. Deeper-penetration OCT imaging demonstrated the internal characteristics of optic disk drusen and their relationship with the lamina cribrosa in vivo. OHND appear as ovoid regions of lower reflectivity bordered by hyperreflective material. Both the larger the drusen are and the more area of the optic canal is occupied by drusen, the greater RNFL abnormalities are associated (Fig. 11).72
The use of autofluorescence (a modality offered on OCT instruments that use a blue scanning laser), or ultrasound scanning are also useful to detect buried OHND. However, EDI-OCT detects ONHD more often and evaluates their shape and structure better than non-EDI OCT and ultrasound B scan.73
Dominant optic atrophy
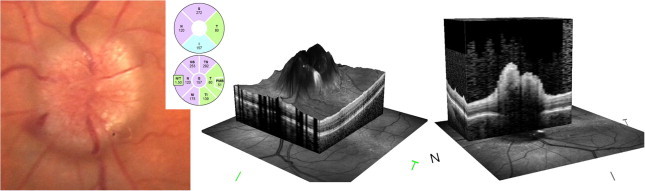
Contrary to glaucoma, in patients with Dominant Optic Atrophy (DOA), RNFL analysis shows a significant temporal preference of RNFL loss followed by the inferior quadrant and a relative sparing of the nasal fibers with a symmetric appearance (Fig. 12).74
Figure 12.
Dominant optic neuropathy. Bilateral temporal pallor and optic disk cupping, with bilateral RNFL loss that predominates in temporal and inferior quadrants. Macular thinning is more pronounced in the corresponding inner and outer nasal and inferior macular quadrants.
There is a significant correlation between average RNFL and GCIPL thickness and VA.75,76
The ONH analysis shows a significantly smaller optic disk area and disk diameters, compared with controls suggesting that patients with DOA are born with fewer optic nerve axons.77
Recently, Barboni et al. studied 39 patients with DOA taking into account the severity of visual loss and the OPA1 mutation type. They reported that the loss of macular retinal ganglion cells (RGC) was the earliest pathological event. Thus, mild cases showed significant macular RGC loss as opposed to substantial maintenance of RNFL thickness, which is significantly decreased only in severe cases and in late stages of the disease.50,78
A clear genotype/phenotype correlation is emerging. RNFL thinning is more pronounced in patients with DOA plus phenotypes that develop additional neuromuscular features.79
Leber optic neuropathy
On the basis of OCT data, Barboni et al. firstly reported that RNFL is thicker than normal in the presymptomatic and early disease states with earliest changes in the temporal and inferior quadrants.80
The temporal fibers (PMB) are the first and most severely affected. In the late period significant pRNFL thinning is present in all sectors.
The late involvement of both superior and nasal quadrants suggests a dynamic evolution of the acute stage that continues for 3 months and may represent a therapeutic window of opportunity.80,81
In childhood-onset Leber optic neuropathy (LHON) (equal or less than 10 years of age) a significant diffuse reduction of RNFL has been demonstrated, whereas a significant reduction of the temporal quadrant was present in the slowly progressive and subclinical LHON cases.82
In unaffected carriers, a thickening of the temporal fibers has also been described. Unaffected males have a more diffuse involvement than females.83
Recently Ramos et al.84 found that the ONH size is larger in LHON carriers than in LHON-affected, suggesting a protective role for this anatomic trait. Such hypothesis is reinforced by the observation that, among the LHON-affected, larger disks correlated with visual recovery and better visual outcome. The findings may be relevant for prognosis.
GCIPL is also diffusely damaged in LHON. More studies will provide information about if early changes are present in carriers and in subacute or early LHNO cases (Fig. 13).
Figure 13.
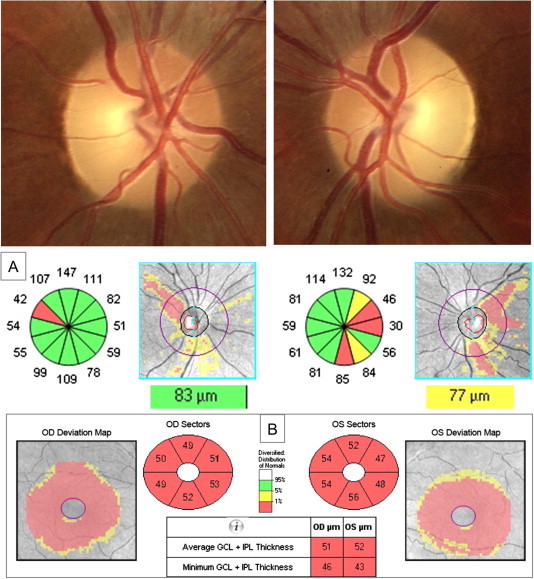
24-Year-old male with Leber optic neuropathy. Fundus examination reveals a bilateral temporal pallor of the optic disk. GCIPL analysis (B) shows a bilateral severe thinning that is more striking than thinning yielded by RNFL analysis (A).
Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of dementia. A recent meta-analysis showed that in AD patients, there was a significant RNFL thickness reduction compared with healthy subjects.85,86 Controversy exists about the correlation between RNFL measurements and the severity of the dementia.87
In addition to RNFL thinning, choroid, macular and GCL thickness are also reduced even without visual failure.87–89
Over the last few decades, earlier stages of AD have been identified , which include mild cognitive impairment (MCI). Early detection is necessary to identify cognitively normal individuals who have a high risk of developing MCI and AD, so that interventions can prevent or delay the progression. Recently, it has been suggested that the detection of the plaques in the retina early in the disease, before the onset of dementia or very early in the course of the disease, and examination with fundus autofluorescence (FAF) and OCT is extremely important and may be a valuable supplement to neurological testing.90
More studies are necessary to assess whether choroidal, RNFL o GCIPL thickness represent an adjunctive biomarker for the diagnosis and follow-up of this pathology.
Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative process that leads to the selective loss of dopaminergic neurons, mainly in the basal ganglia of the brain. Numerous studies have analyzed the ability of OCT to detect abnormalities in PD.91–96
Both RNFL and macular thickness are significantly reduced in patients with PD and correlate with PD severity.95,96
SD-OCT is a valid and reproducible device for detecting subclinical RNFL atrophy in patients with PD, especially the Nsite Axonal Analysis of the Spectralis OCT device. Furthermore, the newer segmentation algorithm of the Spectralis OCT revealed retinal layer atrophy especially in the inner layers of patients with longer disease duration.96
Spund et al. reported that the foveal pit is thinner and broader in PD.97 This remodeling of the foveal architecture raises a novel concept and may represent a visible and quantifiable signature of PD.
Although OCT is a promising quantitative tool in PD research, larger studies are needed before applying it to clinical follow-up.
Other optic neuropathies
OCT can be useful in many other optic neuropathies, for instance to detect early damage in case of retrobulbar origin optic atrophy.
In traumatic optic neuropathy, OCT can reveal RNFL loss from 2 week to 4 weeks after trauma.
In toxic/nutritional-induced optic neuropathies, RNFL thickness may be normal or slightly increased on initial evaluation and become thinned, especially temporally, on future testing.98 The observed thinning of the temporal RNFL corresponds to the more significant effects on the papillo-macular fibers.
Contrary to the predominant temporal RNFL thinning pattern of toxic neuropathies, a recognizable and characteristic form of peripheral retinal atrophy and nasal or “inverse” RNFL loss can occur in children being treated with vigabatrin (Fig. 14).99,100
Figure 14.
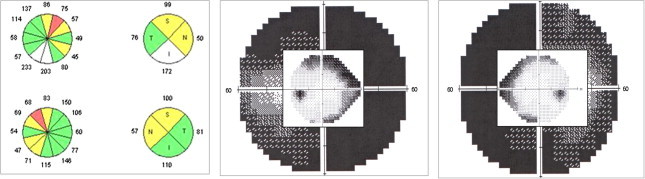
Bilateral and symmetric optic neuropathy due to vigabatrin. Bilateral supero-nasal RNFL thinning and peripheral VF constriction (60-4 and 24-2 strategies are superimposed).
OCT-measured RNFL and GCL are also thinned in compressive optic neuropathy regardless of its etiology.
In optic chiasm syndrome, OCT could quantitatively detect the band atrophy (BA) of the optic disk. The relatively preserved superior and inferior RNFL bundles correlated with the findings of bitemporal hemianopsia.94,95 Both macular and GCL analyses are more useful to detect BA than RNFL analysis (Fig. 15a).96,101,102 Eyes with BA of the optic nerve show significant thinning of the retinal GCL thickness on the nasal macular area, but in authoŕs experience is not uncommon to find a more extended area of GCL thinning compared to VF loss (Fig. 15b).
Figure 15a.
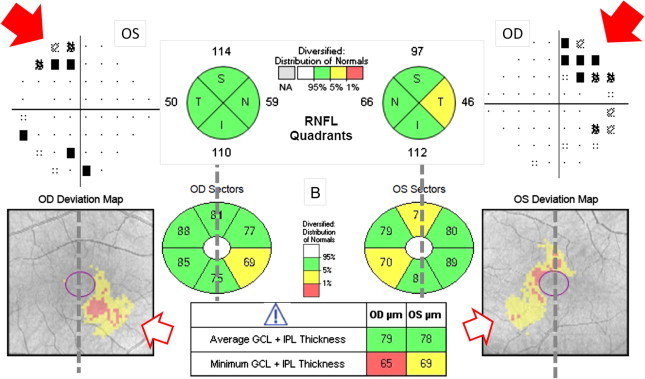
Bitemporal hemianopsia more pronounced in the superior hemifields showing a significant thinning in the inferonasal sectors of both eyes in the GCL analysis (B). The pRNFL thinning does not correspond with the visual field defect as clear as the GCL does.
Figure 15b.
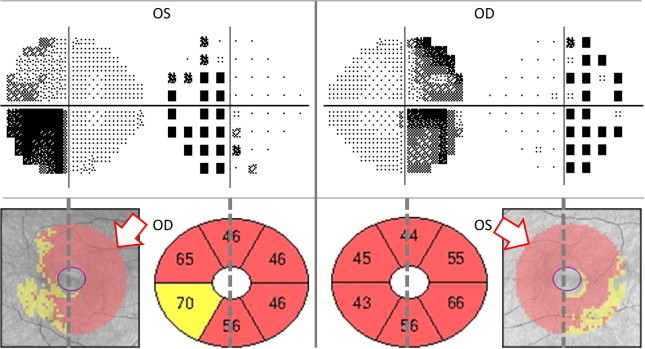
Bitemporal hemianopsia. CGL thinning predominates in the heminasal retina (white arrows), but it extends beyond midline crossing to the temporal hemifield in both eyes.
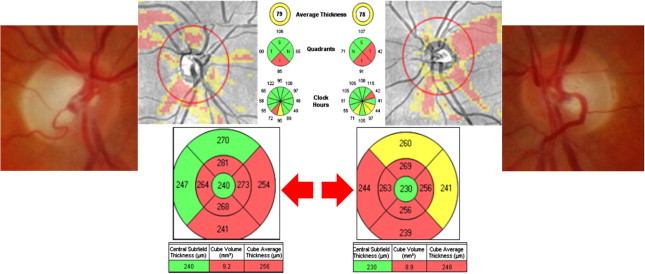
Besides its diagnosis value, in chiasmal tumors, OCT has a predictive role so that the degree of reversibility is related to the preoperative RNFL thickness.103 Patients who have objectively measurable RNFL loss at the time of surgery for chiasmal compressive lesions are less likely to return of VA or VF after surgery. We have observed similar findings in patients following orbital decompression for thyroid eye disease and associated optic neuropathy (Fig. 16).
Figure 16.
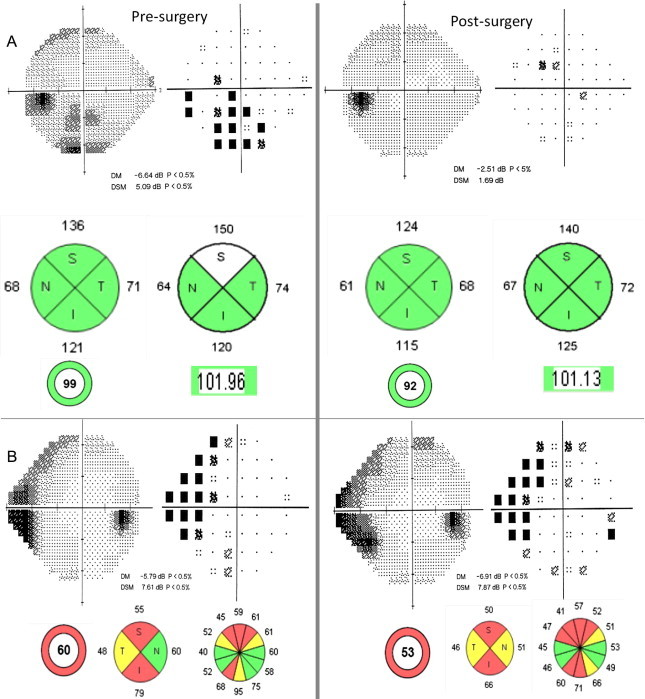
RNFL thickness before orbital decompression for thyroid orbitopathy can predict whether there will be or not visual function recovery after surgery. (A) Patient with Graves orbitopathy showing a RNFL thickness normal despite moderate VF damage preoperatively. Full VF recovery occurred after surgery. (B) Woman with thyroid orbitopathy had both VF and RNFL analysis damaged before surgery, so that no recovery happens after decompressive surgery.
Controversy remains regarding the occurrence of optic nerve degeneration following post-geniculate hemianopsia. Retrograde transsynaptic degeneration of retinal ganglion cell layer (GCL) has been proposed as one of the mechanisms contributing to permanent disability after visual pathway damage104,105 (Fig. 17). Recently, Keller et al. have reported a significant thinning of the GCL in patients with a retrogeniculate lesion.105 The correlation between VF defect and GCL thickness was stronger than that observed between VF and pRNFL, probably because at the macula the neuron bodies are topographically organized to correspond to the VF.
Figure 17.
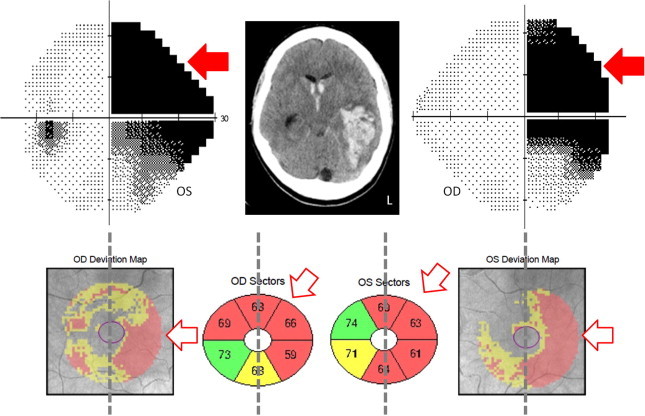
Right homonymous hemianopsia due to an intracranial hemorrhage in the left temporal lobe (shown in the CT scan). A significant GCL thinning is shown in the projecting sector of the retina mapping to the brain lesion, so that the nasal hemiretina of the right eye and the temporal hemiretina of the left eye (contra and ipsilateral to the brain lesion respectively).
These findings provide new insights regarding transsynaptic degeneration of the visual system, supporting the use of GCL thickness as in imaging marker after brain lesions.
Emerging applications and future direction of OCT
Advancements in OCT technology continue to evolve at a quick step. The acquisition of scans is much faster in swept source OCT (SS-OCT) when compared with the SD-OCT and it is very useful for adequate analysis of choroidal thickness and volume in healthy and diseased states.
The commercialization of mobile SD-OCT systems may expand the application spectrum to analyze subjects manifesting significant deteriorated motion, including adult and pediatric patients groups. In fact, hand-held OCT (HH-OCT) has already been used to quantify pRNFL thickness in children with optic pathway gliomas showing a close relationship to visual function.
New emerging functional SD-OCT imaging modalities such as Doppler-SD for blood-flow Imaging and Polarization-Sensitive OCT had a wide spectrum of potential diagnostic applications.
As advanced super-luminescent diodes and femtolaser technologies have become cheaper, commercial ultra-high Resolution OCT machines (approximating 2–3 μm of resolution) will emerge as options to consider.106
OCT technology is changing very quickly and throughout this review there are only some of the multiple possibilities that will emerge in the next future in the field of Neuro-ophthalmology.
Conflict of interest
The authors declared that there is no conflict of interest.
References
- 1.Parisi V., Manni G., Spadaro M., Colacino G., Restuccia R., Marchi S. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–2527. [PubMed] [Google Scholar]
- 2.Galetta K.M., Calabresi P.A., Frohman E.M., Balcer L.J. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8:117–132. doi: 10.1007/s13311-010-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noval S., Contreras I., Muñoz S., Oreja-Guevara C., Manzano B., Rebolleda G. Optical coherence tomography in multiple sclerosis and neuromyelitis optica: an update. Mult Scler Int. 2011;2011:472790. doi: 10.1155/2011/472790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold A., de Boer J.F., Schippling S., Vermersch P., Kardon R., Freen A. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921–932. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 5.Rebolleda G., González-López J.J., Muñoz-Negrete F.J., Oblanca N., Costa-Frossard L., Álvarez-Cermeño J.C. Color-code agreement among stratus, cirrus, and spectralis optical coherence tomography in relapsing-remitting multiple sclerosis with and without prior optic neuritis. Am J Ophthalmol. 2013;155:890–897. doi: 10.1016/j.ajo.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Noval S., Contreras I., Rebolleda G., Muñoz-Negrete F.J. Optical coherence tomography in optic neuritis. Ophthalmology. 2007;114:200. doi: 10.1016/j.ophtha.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Noval S., Contreras I., Rebolleda G., Muñoz-Negrete F.J. Optical coherence tomography versus automated perimetry for follow-up of optic neuritis. Acta Ophthalmol Scand. 2006;84:90–794. doi: 10.1111/j.1600-0420.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 8.Rebolleda G., Noval S., Contreras I., Arnalich-Montiel F., García-Perez J.L., Muñoz-Negrete F.J. Optic disc cupping after optic neuritis evaluated with optic coherence tomography. Eye (Lond) 2009;23:890–894. doi: 10.1038/eye.2008.117. [DOI] [PubMed] [Google Scholar]
- 9.Fisher J.B., Jacobs D.A., Markowitz C.E., Galetta S.L., Volpe N.J. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Scheel M., Finke C., Oberwahrenbrock T., Freing A., Pech L.M., Schlichting J. Retinal nerve fibre layer thickness correlates with brain white matter damage in multiple sclerosis: A combined optical coherence tomography and diffusion tensor imaging study. Mult Scler. 2014 doi: 10.1177/1352458514535128. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Fjeldstad C., Bemben M., Pardo G. Reduced retinal nerve fiber layer and macular thickness in patients with multiple sclerosis with no history of optic neuritis identified by the use of spectral domain high-definition optical coherence tomography. J Clin Neurosci. 2011;18:1469–1472. doi: 10.1016/j.jocn.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Martin E., Pueyo V., Pinilla I., Ara J.R., Martin J., Fernández J. Fourier-domain OCT in multiple sclerosis patients: reproducibility and ability to detect retinal nerve fiber layer atrophy. Invest Ophthalmol Vis Sci. 2011;52:4124–4131. doi: 10.1167/iovs.10-6643. [DOI] [PubMed] [Google Scholar]
- 13.Rebolleda G., García-García A., Won Kim H.R., Muñoz-Negrete F.J. Comparison of retinal nerve fiber layer measured by time domain and spectral domain optical coherence tomography in optic neuritis. Eye. 2011;25:233–238. doi: 10.1038/eye.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leal-Fonseca M., Rebolleda G., Oblanca N., Moreno-Montañes J., Muñoz-Negrete F.J. A comparison of false positives in retinal nerve fiber layer, optic nerve head and macular ganglion cell-inner plexiform layer from two spectral-domain optical coherence tomography devices. Graefes Arch Clin Exp Ophthalmol. 2014;252:321–330. doi: 10.1007/s00417-013-2529-7. [DOI] [PubMed] [Google Scholar]
- 15.Walter S.D., Ishikawa H., Galetta K.M., Sakai R.E., Feller D.J., Henderson S.B. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saidha S., Sotirchos E.S., Oh J., Syc S.B., Seigo M.A., Shiee N. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70:34–43. doi: 10.1001/jamaneurol.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht P., Ringelstein M., Müller A.K., Keser N., Dietlein T., Lappas A. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler. 2012;18:1422–1429. doi: 10.1177/1352458512439237. [DOI] [PubMed] [Google Scholar]
- 18.Saidha S., Syc S.B., Durbin M.K., Eckstein C., Oakley J.D., Meyer S.A. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17:1449–1463. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Martin E., Polo V., Larrosa J.M., Marques M.L., Herrero R., Martin J. Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology. 2014;121:573–579. doi: 10.1016/j.ophtha.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez JJ, Rebolleda G, Leal M, Oblanca N, Muñoz-Negrete FJ, Costa-Frossard L, Alvarez-Cermeño JC. Comparative diagnostic accuracy of ganglion cell-inner plexiform and retinal nerve fiber layer thickness measures by Cirrus and Spectralis OCTs in relapsing-remitting multiple sclerosis. Biomed Res Int 2014; in press. [DOI] [PMC free article] [PubMed]
- 21.Syc S.B., Saidha S., Newsome S.D., Ratchford J.N., Levy M., Ford E. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sotirchos E.S., Seigo M.A., Calabresi P.A., Saidha S. Comparison of point estimates and average thicknesses of retinal layers measured using manual optical coherence tomography segmentation for quantification of retinal neurodegeneration in multiple sclerosis. Curr Eye Res. 2013;38:224–228. doi: 10.3109/02713683.2012.722243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello F., Hodge W., Pan Y.I., Eggenberger E., Coupland S., Kardon R.H. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14:893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- 24.Costello F., Coupland S., Hodge W. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 25.Narayanan D., Cheng H., Bonem K.N., Saenz R., Tang R.A., Frishman L.J. Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler. 2014 doi: 10.1177/1352458514523498. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Martin E., Pueyo V., Martin J., Almarcegui C., Ara J.R., Dolz I. Progressive changes in the retinal nerve fiber layer in patients with multiple sclerosis. Eur J Ophthalmol. 2010;20:167–173. doi: 10.1177/112067211002000123. [DOI] [PubMed] [Google Scholar]
- 27.Ratchford J.N., Saidha S., Sotirchos E.S., Oh J.A., Seigo M.A., Eckstein E. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80:47–54. doi: 10.1212/WNL.0b013e31827b1a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarbin M.A., Jampol L.M., Jager R.D., Reder A.T., Francis G., Collins W. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology. 2013;120:1432–1439. doi: 10.1016/j.ophtha.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand J.M., Nolan R., Schwartz D.M., Graves J., Green A.J. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(Pt 6):1786–1793. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saidha S., Sotirchos E.S., Ibrahim M.A., Crainiceanu C.M., Gelfand J.M., Sepah Y.J. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abegg M., Dysli M., Wolf S., Kowal J., Dufour P., Zinkernagel M. Microcystic macular edema: Retrograde maculopathy caused by optic neuropathy. Ophthalmology. 2014;121:142–149. doi: 10.1016/j.ophtha.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 32.Pulicken M., Gordon-Lipkin E., Balcer L.J., Frohman E., Cutter G., Calabresi P.A. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–2092. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro M.L., Fernandes D.B., Apóstolos-Pereira S.L., Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;26(53):3959–3966. doi: 10.1167/iovs.11-9324. [DOI] [PubMed] [Google Scholar]
- 34.Naismith R.T., Tutlam N.T., Xu J., Klawiter E.C., Shepherd J., Trinkaus K. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72:1077–1082. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratchford J.N., Quigg M.E., Conger A., Frohman T., Frohman E., Balcer L.J. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302–308. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider E., Zimmermann H., Oberwahrenbrock T., Kaufhold F., Kadas E.M., Petzold A. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One. 2013;8(6):e66151. doi: 10.1371/journal.pone.0066151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes D.B., Raza A.S., Nogueira R.G., Wang D., Callegaro D., Hood D.C. Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology. 2013;120:387–394. doi: 10.1016/j.ophtha.2012.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello F, Pan YI, Burton J, Chan W, Hodge W. Predicting visual recovery after optic neuritis. In: 40th NANOS (North American Neuro-Ophthalmology Society) meeting, Rio Grande, Puerto Rico, USA: Annual Meeting Syllabus; 2014. p. 319.
- 39.Garas A., Simó M., Holló G. Nerve fiber layer and macular thinning measured with different imaging methods during the course of acute optic neuritis. Eur J Ophthalmol. 2011;21:473–483. doi: 10.5301/EJO.2010.5844. [DOI] [PubMed] [Google Scholar]
- 40.Rebolleda G, de Dompablo E, Muñoz-Negrete J. Ganglion cell layer analysis unmasks axonal loss in anterior optic neuritis. J Neurophthalmol 2014; in press. [DOI] [PubMed]
- 41.Saidha S., Syc S.B., Ibrahim M.A., Eckstein C., Warner C.V., Farrell S.K. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134:518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Jia Y., Spain R., Potsaid B., Liu J.J., Baumann B. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. 2014 doi: 10.1136/bjophthalmol-2013-304547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farsiu S., Izatt J.A., Toth C.A. Advances and emerging applications of spectral-domain. Optical coherence tomography imaging in ophthalmology. US Ophthalmic Rev. 2007;3:14–17. [Google Scholar]
- 44.Contreras I., Noval S., Rebolleda G., Muñoz-Negrete F.J. Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology. 2007;114:2338–2344. doi: 10.1016/j.ophtha.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 45.Kernstock C., Beisse F., Wiethoff S., Mast A., Krapp E., Grund R. Assessment of functional and morphometric endpoints in patients with non-arteritic anterior ischemic optic neuropathy (NAION) Graefes Arch Clin Exp Ophthalmol. 2014;252:515–521. doi: 10.1007/s00417-014-2572-z. [DOI] [PubMed] [Google Scholar]
- 46.Dotan G., Goldstein M., Kesler A., Skarf B. Long-term retinal nerve fiber layer changes following nonarteritic anterior ischemic optic neuropathy. Clin Ophthalmol. 2013;7:735–740. doi: 10.2147/OPTH.S42522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contreras I., Rebolleda G., Noval S. Optic disc evaluation by optical coherence tomography in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2007;48:4087–4092. doi: 10.1167/iovs.07-0171. [DOI] [PubMed] [Google Scholar]
- 48.Contreras I., Rebolleda G., Noval S., Muñoz-Negrete F.J. Ischemic optic neuropathy. Ophthalmology. 2009;116:814. doi: 10.1016/j.ophtha.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Fernández-Buenaga R., Rebolleda G., Muñoz-Negrete F.J., Contreras I., Casas-Llera P. Macular thickness. Ophthalmology. 2009;116:1587. doi: 10.1016/j.ophtha.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 50.Rönnbäck C., Milea D., Larsen M. Imaging of the macula indicates early completion of structural deficit in autosomal-dominant optic atrophy. Ophthalmology. 2013;120:2672–2677. doi: 10.1016/j.ophtha.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Bellusci C., Savini G., Carbonelli M., Carelli V., Sadun A.A., Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol. 2008;246:641–647. doi: 10.1007/s00417-008-0767-x. [DOI] [PubMed] [Google Scholar]
- 52.Papchenko T., Grainger B.T., Savino P.J., Gamble G.D., Danesh-Meyer H.V. Macular thickness predictive of visual field sensitivity in ischaemic optic neuropathy. Acta Ophthalmol. 2012;90:463–469. doi: 10.1111/j.1755-3768.2012.02467.x. [DOI] [PubMed] [Google Scholar]
- 53.Gonul S., Koktekir B.E., Bakbak B., Gedik S. Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2013;97:1045–1050. doi: 10.1136/bjophthalmol-2013-303438. [DOI] [PubMed] [Google Scholar]
- 54.Hedges T.R., 3rd, Vuong L.N., Gonzalez-Garcia A.O., Mendoza-Santiesteban C.E., Amaro-Quierza M.L. Subretinal fluid from anterior ischemic optic neuropathy demonstrated by optical coherence tomography. Arch Ophthalmol. 2008;126:812–815. doi: 10.1001/archopht.126.6.812. [DOI] [PubMed] [Google Scholar]
- 55.Rebolleda G., Pérez-López M., Casas-LLera P., Contreras I., Muñoz-Negrete F.J. Visual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroids. Graefes Arch Clin Exp Ophthalmol. 2013;251:255–260. doi: 10.1007/s00417-012-1995-7. [DOI] [PubMed] [Google Scholar]
- 56.Rebolleda G., Muñoz-Negrete F.J. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:5197–5200. doi: 10.1167/iovs.08-2528. [DOI] [PubMed] [Google Scholar]
- 57.Scott C.J., Kardon R.H., Lee A.G., Frisén L., Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128:705–711. doi: 10.1001/archophthalmol.2010.94. [DOI] [PubMed] [Google Scholar]
- 58.Wang J.K., Kardon R.H., Kupersmith M.J., Garvin M.K. Automated quantification of volumetric optic disc swelling in papilledema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:4069–4075. doi: 10.1167/iovs.12-9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NORDIC Idiopathic Intracranial Hypertension Study Group The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014;71:693–701. doi: 10.1001/jamaneurol.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dattilo M, Olumba K, Elmalem VI. Macular outer retinal abnormalities in severe papilledema. In: 40th NANOS (North American Neuro-Ophthalmology Society) meeting, Rio Grande, Puerto Rico, USA: Annual Meeting Syllabus; 2014. p. 307.
- 61.Sibony P., Kupersmith M., Rohlf F.J. Geometric morphometrics of the peripapillary SD-OCT: Shape analysis of the RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2011;52:7987–7995. doi: 10.1167/iovs.11-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kupersmith M., Sibony P., Mandel G., Durbin M., Kardon R. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary RPE layer in papilledema. Inv Ophthalmol Vis Sci. 2011;52:6558–6564. doi: 10.1167/iovs.10-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia Y., Morrison J.C., Tokayer J., Tan O., Lombardi L., Baumann B. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3:3127–3137. doi: 10.1364/BOE.3.003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gili P., Flores-Rodríguez P., Martin-Ríos M.D., Carrasco Font C. Anatomical and functional impairment of the nerve fiber layer in patients with optic nerve head drusen. Graefes Arch Clin Exp Ophthalmol. 2013;251:2421–2428. doi: 10.1007/s00417-013-2438-9. [DOI] [PubMed] [Google Scholar]
- 65.Noval S., Visa J., Contreras I. Visual field defects due to optic disk drusen in children. Graefes Arch Clin Exp Ophthalmol. 2013;251:2445–2450. doi: 10.1007/s00417-013-2384-6. [DOI] [PubMed] [Google Scholar]
- 66.Roh S., Noecker R.J., Schuman J.S., Hedges T.R., 3rd, Weiter J.J., Mattox C. Effect of optic nerve head drusen on nerve fiber layer thickness. Ophthalmology. 1998;105:878–885. doi: 10.1016/S0161-6420(98)95031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casado A, Rebolleda G, Guerrero L, Leal M, Contreras I, Oblanca N, Muñoz-Negrete FJ. Measurement of retinal nerve fiber layer and macular ganglion cell-inner plexiform layer with spectral-domain optical coherence tomography in patients with optic nerve head drusen. Graefes Arch Clin Exp Ophthalmol 2014; [Epub ahead of print]. [DOI] [PubMed]
- 68.Johnson L.N., Diehl M.L., Hamm C.W., Sommerville D.N., Petroski G.F. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127:45–49. doi: 10.1001/archophthalmol.2008.524. [DOI] [PubMed] [Google Scholar]
- 69.Flores-Rodríguez P., Gili P., Martín-Ríos M.D. Sensitivity and specificity of time-domain and spectral-domain optical coherence tomography in differentiating optic nerve head drusen and optic disc oedema. Ophthalmic Physiol Opt. 2012;32:213–221. doi: 10.1111/j.1475-1313.2012.00902.x. [DOI] [PubMed] [Google Scholar]
- 70.Kulkarni K.M., Pasol J., Rosa P.R., Lam B.L. Differentiating mild papilledema and buried optic nerve head drusen using spectral domain optical coherence tomography. Ophthalmology. 2014;121:959–963. doi: 10.1016/j.ophtha.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K.M., Woo S.J., Hwang J.M. Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:971–977. doi: 10.1016/j.ophtha.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Sato T., Mrejen S., Spaide R.F. Multimodal imaging of optic disc drusen. Am J Ophthalmol. 2013;156:275–282. doi: 10.1016/j.ajo.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 73.Merchant K.Y., Su D., Park S.C., Qayum S., Banik R., Liebmann J.M. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology. 2013;120:1409–1414. doi: 10.1016/j.ophtha.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 74.Kim T.W., Hwang J.M. Stratus OCT in dominant optic atrophy: features differentiating it from glaucoma. J Glaucoma. 2007;16:655–658. doi: 10.1097/IJG.0b013e31804d23aa. [DOI] [PubMed] [Google Scholar]
- 75.Yu-Wai-Man P., Bailie M., Atawan A., Chinnery P.F., Griffiths P.G. Pattern of retinal ganglion cell loss in dominant optic atrophy due to OPA1 mutations. Eye. 2011;25:596–602. doi: 10.1038/eye.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russo A., Delcassi L., Marchina E., Semeraro F. Correlation between visual acuity and OCT-measured retinal nerve fiber layer thickness in a family with ADOA and an OPA1 mutation. Ophthalmic Genet. 2013;34:69–74. doi: 10.3109/13816810.2012.702259. [DOI] [PubMed] [Google Scholar]
- 77.Barboni P., Carbonelli M., Savini G., Foscarini B., Parisi V., Valentino M.L. OPA1 mutations associated with dominant optic atrophy influence optic nerve head size. Ophthalmology. 2010;117:1547–1553. doi: 10.1016/j.ophtha.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 78.Barboni P., Savini G., Parisi V., Carbonelli M., La Morgia C., Maresca A. Retinal nerve fiber layer thickness in dominant optic atrophy measurements by optical coherence tomography and correlation with age. Ophthalmology. 2011;118:2076–2080. doi: 10.1016/j.ophtha.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 79.Barboni P., Savini G., Cascavilla M.L., Caporali L., Milesi J., Borrelli E. Early macular retinal ganglion cell loss in dominant optic atrophy: genotype-phenotype correlation. Am J Ophthalmol. 2014 doi: 10.1016/j.ajo.2014.05.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Barboni P., Savini G., Valentino M.L., Montagna P., Cortelli P., De Negri A.M. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112:120–126. doi: 10.1016/j.ophtha.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 81.Barboni P., Carbonelli M.., Savini G., Ramos Cdo V., Carta A., Berezovsky A. Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–627. doi: 10.1016/j.ophtha.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 82.Barboni P., Savini G., Valentino M.L., La Morgia C., Bellusci C., De Negri A.M. Leber’s hereditary optic neuropathy with childhood onset. Invest Ophthalmol Vis Sci. 2006;47:5303–5309. doi: 10.1167/iovs.06-0520. [DOI] [PubMed] [Google Scholar]
- 83.Savini G., Barboni P., Valentino M.L., Montagna P., Cortelli P., De Negri A.M. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology. 2005;112:127–131. doi: 10.1016/j.ophtha.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 84.Ramos Cdo V., Bellusci C., Savini G., Carbonelli M., Berezovsky A., Tamaki C. Association of optic disc size with development and prognosis of Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2009;50:1666–1674. doi: 10.1167/iovs.08-2695. [DOI] [PubMed] [Google Scholar]
- 85.He X.F., Liu Y.T., Peng C., Zhang F., Zhuang S., Zhang J.S. Optical coherence tomography assessed retinal nerve fiber layer thickness in patients with Alzheimer’s disease: a meta-analysis. Int J Ophthalmol. 2012;5:401–405. doi: 10.3980/j.issn.2222-3959.2012.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larrosa J.M., Garcia-Martin E., Bambo M.P., Pinilla J., Polo V., Otin S. Potential new diagnostic tool for Alzheimer’s disease using a linear discriminant function for Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:3043–3051. doi: 10.1167/iovs.13-13629. [DOI] [PubMed] [Google Scholar]
- 87.Gharbiya M., Trebbastoni A., Parisi F., Manganiello S., Cruciani F., D’Antonio F. Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimer’s Dis. 2014;40:907–917. doi: 10.3233/JAD-132039. [DOI] [PubMed] [Google Scholar]
- 88.Marziani E., Pomati S., Ramolfo P., Cigada M., Giani A., Mariani C. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5953–5958. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 89.Moschos M.M., Markopoulos I., Chatziralli I., Rouvas A., Papageorgiou S.G., Ladas I. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:782–788. doi: 10.2174/156720512802455340. [DOI] [PubMed] [Google Scholar]
- 90.Sergott RC, Kayabasi U. Progression of plaques in retina with dementia in Alzheimeŕs disease. In: 40th NANOS (North American Neuro-Ophthalmology Society) meeting, Rio Grande, Puerto Rico, USA: Annual Meeting Syllabus; 2014. p. 148.
- 91.Inzelber R., Ramirez J.A., Nisipeanu P., Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004;44:2793–2797. doi: 10.1016/j.visres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Kirbas S., Turkyilmaz K., Tufekci A., Durmus M. Retinal nerve fiber layer in parkinson disease. J Neuroophthalmol. 2013;33:62–65. doi: 10.1097/WNO.0b013e3182701745. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Martin E., Satue M., Fuertes I., Otin S., Alarcia R., Herrero R. Ability and reproducibility of Fourier-domain Optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology. 2012;119:2161–2167. doi: 10.1016/j.ophtha.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Yu J.G., Feng Y.F., Xiang Y., Huang J.H., Savini G., Parisi V. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014;9(1):e85718. doi: 10.1371/journal.pone.0085718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Satue M., Seral M., Otin S., Alarcia R., Herrero R., Bambo M.P. Retinal thinning and correlation with functional disability in patients with Parkinson’s disease. Br J Ophthalmol. 2014;98:350–355. doi: 10.1136/bjophthalmol-2013-304152. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Martin E., Larrosa J.M., Polo V., Satue M., Marques M.L., Alarcia R. Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am J Ophthalmol. 2014;157:470–478. doi: 10.1016/j.ajo.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 97.Spund B., Ding Y., Liu T. Remodeling of the fovea in Parkinson disease. J Neural Transm. 2013;120:745–753. doi: 10.1007/s00702-012-0909-5. [DOI] [PubMed] [Google Scholar]
- 98.Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Curr Opin Ophthalmol. 2011;22:124–132. doi: 10.1097/ICU.0b013e328343c1a3. [DOI] [PubMed] [Google Scholar]
- 99.Buncic J.R., Westall C.A., Panton C.M., Munn J.R., MacKeen L.D., Logan W.J. Characteristic retinal atrophy with secondary “inverse” optic atrophy identifies vigabatrin toxicity in children. Ophthalmology. 2004;111:1935–1942. doi: 10.1016/j.ophtha.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebolleda G., García Pérez J.L., Muñoz Negrete F.J., Tang R.A. Vigabatrin toxicity in children. Ophthalmology. 2005;112:1322–1323. doi: 10.1016/j.ophtha.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 101.Costa-Cunha L.V., Cunha L.P., Malta R.F., Monteiro M.L. Comparison of Fourier domain and time-domain optical coherence tomography in the detection of band atrophy of the optic nerve. Am J Ophthalmol. 2009;147:56–63. doi: 10.1016/j.ajo.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 102.Moura F.C., Medeiros F.A., Monteiro M.L. Evaluation of macular thickness measurements for detection of band atrophy of the optic nerve using optical coherence tomography. Ophthalmology. 2007;114:175–181. doi: 10.1016/j.ophtha.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 103.Danesh-Meyer H.V., Papchenko T., Savino P.J., Law A., Evans J., Gamble G.D. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci. 2008;49:1879–1885. doi: 10.1167/iovs.07-1127. [DOI] [PubMed] [Google Scholar]
- 104.Jindahra P., Petrie A., Plant G.T. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135(Pt 2):534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 105.Keller J., Sánchez-Dalmau B.F., Villoslada P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion loss. PLoS One. 2014;9(5):e97444. doi: 10.1371/journal.pone.0097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walsh AC. Next-generation OCT: what to look for in a Fourier domain OCT instrument. Retinal Physician 2007. Available from: http://www.retinalphysician.com/articleviewer.aspx?articleID=100302.


