Abstract
BACKGROUND:
Despite guideline recommendations, patients suspected of having COPD often are treated empirically instead of undergoing spirometry to confirm airflow obstruction (AFO). Accurate diagnosis and treatment are essential to provide high-quality, value-oriented care. We sought to identify predictors associated with AFO among patients with and treated for COPD prior to performance of confirmatory spirometry.
METHODS:
We identified a cohort of veterans with spirometry performed at Pacific Northwest Department of Veterans Affairs medical centers between 2003 and 2007. We included only patients with empirically diagnosed COPD in the 2 years prior to spirometry who were also taking inhaled medication to treat COPD in the 1 year prior to spirometry. We used relative risk regression analysis to identify predictors of AFO.
RESULTS:
Among patients empirically treated for COPD (N = 3,209), 62% had AFO. Risk factors such as older age, prior smoking status, and underweight status were associated with AFO on spirometry. In contrast, comorbidities often associated with somatic symptoms were associated with absence of AFO and included congestive heart failure, depression, diabetes, obesity, and sleep apnea.
CONCLUSIONS:
Comorbidities associated with somatic complaints of dyspnea were associated with a lower risk of having airflow limitations, suggesting that empirical diagnosis and treatment of COPD may lead to inappropriate treatment of individuals who do not have AFO.
Patients presenting with dyspnea often are given a diagnosis of and treated for COPD prior to obtaining confirmatory spirometry, particularly if they are current or past smokers. Despite endorsement by the National Quality Forum and multiple professional societies, spirometry often is underused to confirm the presence of fixed airflow obstruction (AFO) and to properly diagnose COPD.1‐6 This gap between National Quality Forum recommendations and practice is likely driven by attitudes and beliefs about the lack of clinical utility of spirometry in the management of patients with COPD.7‐10 Empirical COPD treatment in patients without COPD represents a potential lost opportunity to identify and treat the true etiology of symptoms and could lead to adverse effects from unnecessary medications or delays in appropriate management.11‐13 We sought to examine the frequency at which patients given an empirical diagnosis of and treated for COPD were subsequently found to have AFO on spirometry as well as the factors predictive of AFO.
Materials and Methods
Study Design, Setting, and Subjects
We used data collected from a cohort of US veterans receiving care at one of three Pacific Northwest Department of Veterans Affairs (VA) medical centers. We identified patients who had their first spirometry between January 2003 and December 2007. The index date was defined as the date of first spirometry. The Institutional Review Board of the VA Puget Sound Healthcare System approved this study (IRB project approval number 01386).
Inclusion Criteria:
We defined empirical diagnosis of COPD within 2 years prior to the index date as (1) two or more outpatient visits with an International Classification of Diseases, Ninth Revision (ICD-9), code for COPD (491.X, 492.X, 493.2, 496.X); (2) an inpatient COPD exacerbation defined as a primary ICD-9 discharge diagnosis of COPD; or (3) an outpatient COPD exacerbation defined as a visit for COPD and a prescription for either an oral antibiotic or an oral steroid within 2 days of the clinic visit. Among patients receiving a diagnosis of COPD, we defined empirical treatment of COPD as any prescription for a short-acting β-agonist, ipratropium, long-acting β-agonist (LABA), or inhaled corticosteroid (ICS) in the year prior to the index date. Data on tiotropium use were not collected because tiotropium was not on the VA formulary at the time of the study.
Exclusion Criteria:
We excluded veterans who did not have complete spirometry and those with lung cancer (ICD-9 codes 162.X or 163.X). We also excluded patients with asthma (ICD-9 codes 593, 493.0, 493.1, 493.8, and 493.9) to avoid potential misclassification.
Data Collection
We collected data from the VISN 20 Data Warehouse, a regional relational database of clinical and nonclinical data. Spirometry results were obtained from the data warehouse or from direct interrogation of the pulmonary function testing equipment.
Outcomes
We defined AFO as a postbronchodilator FEV1/FVC below the lower limit of normal as recommended by the American Thoracic Society.14 As part of clinical protocol, patients were given bronchodilators if they had AFO on prebronchodilator spirometry unless they reported taking a bronchodilator within 2 h of the test. For this reason, we substituted the prebronchodilator value when the postbronchodilator value was not available (12.3% of patients did not have postbronchodilator spirometry). As described herein, we also performed sensitivity analyses restricted to patients who had both prebronchodilator and postbronchodilator testing.
Exposures
The exposures of interest were race, active smoking in the prior year; sex; site of care; age; BMI; and the following six comorbidities defined by ICD-9 codes in the 12 months prior to spirometry: acute coronary syndrome, congestive heart failure (CHF), depression, diabetes, sleep apnea, and hypertension (HTN). Patients were categorized as underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI ≤ 24.99 kg/m2), overweight (25 kg/m2 < BMI ≤ 29.99 kg/m2), or obese (BMI ≥ 30 kg/m2) according to the World Health Organization classification.15 Site of care was one of three sites in the Pacific Northwest: one nonacademic center in a rural setting, one rural academic center, and one tertiary referral center for a major metropolitan area.
We also assessed the total number of COPD exacerbations (both inpatient and outpatient) in the 2 years prior to the index date. Inpatient COPD exacerbations were defined as a primary discharge diagnosis of COPD, and outpatient exacerbations were defined as an outpatient COPD diagnosis accompanied by a prescription of an antibiotic or oral steroid within 2 days of the clinic visit.
Statistical Analysis
Because AFO was common, we used a modified Poisson regression approach to allow estimation of the relative risk (RR) of the outcome. All covariates were included in a single model. Stata 12.0 software was used for all analyses.16 Additional analyses were repeated using GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria for AFO (FEV1/FVC < 0.70). We performed a sensitivity analysis among a restricted cohort of veterans with clinically diagnosed COPD who had received more-intensive inhaled therapy for COPD, which we defined as a prescription of six or more albuterol canisters, three or more ipratropium canisters, one or more LABAs, or one or more ICSs in the prior year. We performed an additional sensitivity analysis restricting the cohort to only patients who had both prebronchodilator and postbronchodilator spirometry.
Results
Description of the Cohort
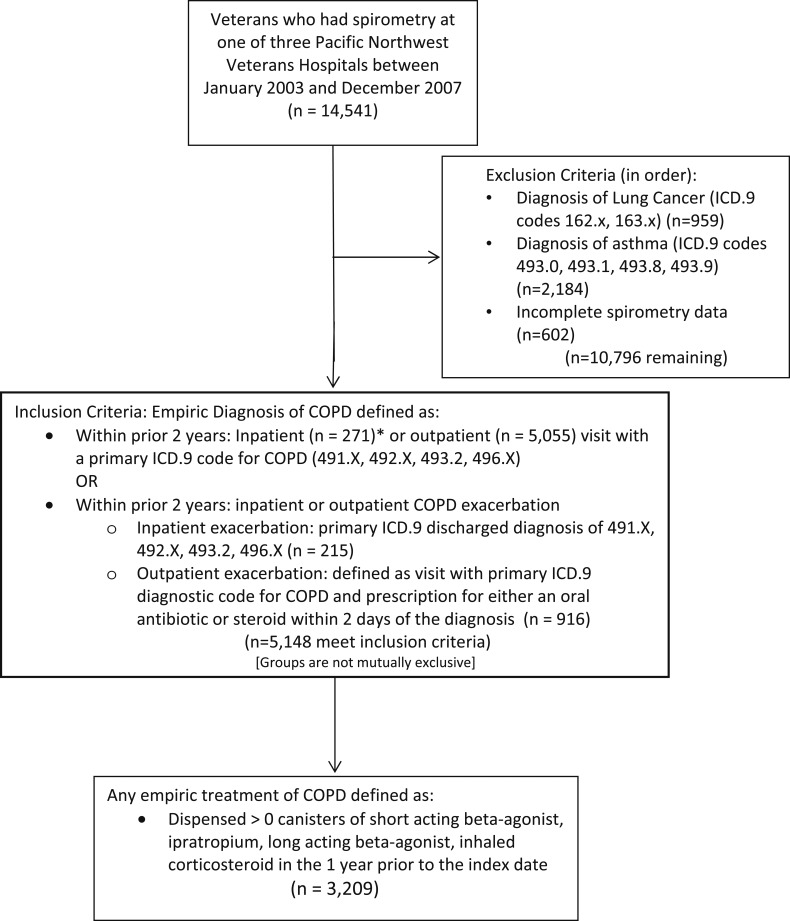
There were 14,541 veterans who had spirometry at one of three VA medical centers during the study period, 5,148 (35.4%) of whom had a prior diagnosis of COPD. Among patients with a clinical diagnosis of COPD, approximately one-half (n = 2,830 [55%]) had evidence of AFO on spirometry. Of patients with a clinical diagnosis of COPD, 3,209 (62.3%) were also treated with inhaled medications (Fig 1). Table 1 shows the characteristics of patients treated for COPD with and without AFO by spirometry. Among patients who received treatment for COPD, 62% (n = 1,996) had AFO.
Figure 1 –
Cohort selection. ICD.9 = International Classification of Diseases, Ninth Revision.
TABLE 1 ] .
Characteristics of Patients Given an ICD-9 Diagnosis of COPD on Any Inhaled Medication by Presence of Airflow Obstruction (LLN) on Spirometry
| Covariate | Airflow Obstruction (n = 1,996) | No Airflow Obstruction (n = 1,213) | P Value |
| White race | 77.8 | 82.1 | .003 |
| Smoking in prior year | 46.7 | 41.1 | .002 |
| Male sex | 97.3 | 96.1 | .05 |
| Site | < .001 | ||
| Rural clinical center (n = 956) | 34.5 | 65.5 | |
| Tertiary center (n = 1,770) | 43.8 | 56.2 | |
| Rural academic center (n = 483) | 22.4 | 77.6 | |
| Age, y | 66.3 ± 9.9 | 65.3 ± 11.0 | .01 |
| BMI | < .001 | ||
| Underweight | 4.2 | 0.9 | |
| Normal weight | 30.4 | 15.3 | |
| Overweight | 35.5 | 30.8 | |
| Obese | 30.0 | 53.1 | |
| Comorbidities in prior year | |||
| ACS | 2.6 | 3.5 | .13 |
| CHF | 12.7 | 20.7 | < .001 |
| Depression | 20.3 | 25.6 | < .001 |
| Diabetes | 16.8 | 31.0 | < .001 |
| Sleep apnea | 6.0 | 12.9 | < .001 |
| HTN | 54.4 | 66.5 | < .001 |
| Number of comorbidities | 1.13 ± 1.0 | 1.60 ± 1.14 | < .001 |
| Exacerbations in prior 2 y | 0.65 ± 1.5 | 0.32 ± 0.81 | < .001 |
Data are presented as % or mean ± SD. ACS = acute coronary syndrome; CHF = congestive heart failure; HTN = hypertension; ICD-9 = International Classification of Diseases, Ninth Revision; LLN = lower limit of normal.
Among patients given a diagnosis of and treated for COPD, those with AFO were older (mean age, 66.3 years vs 65.3 years; P = .01), were more likely to have smoked in the prior year (46.7% vs 41.1%, P ≤ .01), and were more likely to be underweight (4.2% vs 0.9%, P < .01). They also had a greater mean number of clinically diagnosed exacerbations in the prior year (0.65 vs 0.32, P < .01). Patients who had AFO were less likely to have CHF, depression, diabetes, sleep apnea, or HTN in the year prior to the index date than those without AFO. The mean number of selected comorbidities among patients with AFO was lower at 1.13 compared with 1.60 among those without AFO. Among patients with clinically diagnosed COPD taking inhaled medications, 65.5% seen at the rural clinical center had AFO compared with 56.2% seen at the tertiary referral center and 77.6% at the rural academic center (P < .01) (Table 1).
RR of AFO Among Patients Given a Diagnosis of and Treated for COPD
AFO was more likely among patients who had smoked in the prior year (RR, 1.08; 95% CI, 1.02-1.15). These patients were more likely to be older (RR, 10.04 per decade; 95% CI, 10.01-10.07) and underweight (RR, 1.16; 95% CI, 1.06-1.26) (Table 2). Patients with a greater number of exacerbations in the 2 years prior to the index date had a higher RR of AFO (RR, 1.06; 95% CI, 1.04-1.08). Patients seen at the rural academic center were more likely to have AFO than those seen at the rural clinical center (RR, 1.18; 95% CI, 1.10-1.27), whereas those seen at the tertiary center were less likely to have AFO (RR, 0.86; 95% CI, 0.81-0.91).
TABLE 2 ] .
RR of Airflow Obstruction on Spirometry Among Patients Given an ICD-9 Diagnosis of COPD on Empirical Treatment by Demographics and Comorbidities (N = 3,209)
| Covariatea | RR (95% CI) | P Value |
| White race | 0.98 (0.92-1.04) | .58 |
| Smoking in prior year | 1.08 (1.02-1.15) | .006 |
| Site of care | ||
| Rural clinical center | Reference | … |
| Tertiary center | 0.86 (0.81-0.91) | < .01 |
| Rural academic center | 1.18 (1.10-1.27) | < .01 |
| Age (per decade) | 10.04 (10.01-10.07) | .01 |
| BMI | ||
| Normal weight | Reference | … |
| Underweight | 1.16 (1.06-1.26) | .001 |
| Overweight | 0.89 (0.84-0.94) | < .001 |
| Obese | 0.71 (0.66-0.76) | < .001 |
| ACS | 0.99 (0.83-1.19) | .92 |
| CHF | 0.84 (0.76-0.92) | < .01 |
| Depression | 0.90 (0.84-0.96) | .002 |
| Diabetes | 0.85 (0.78-0.92) | < .01 |
| Sleep apnea | 0.85 (0.74-0.98) | .02 |
| HTN | 0.91 (0.86-0.96) | < .01 |
| Prior exacerbationsb | 1.06 (1.04-1.08) | < .01 |
RR = relative risk. See Table 1 legend for expansion of other abbreviations.
All covariates were included in a single model.
Number of COPD exacerbations in the 2 y prior to the index date.
Comorbid illnesses were generally associated with a lower risk of having AFO. Compared with normal weight patients, overweight (RR, 0.89; 95% CI, 0.84-0.94) and obese (RR, 0.71; 95% CI, 0.66-0.76) patients were less likely to have AFO. Patients with CHF (RR, 0.84; 95% CI, 0.76-0.92), depression (RR, 0.90; 95% CI, 0.84-0.96), diabetes (RR, 0.85; 95% CI, 0.78-0.92), sleep apnea (RR, 0.85; 95% CI, 0.74-0.98), and HTN (RR, 0.91; 95% CI, 0.86-0.96) were also less likely to have AFO. Patients with a greater number of comorbidities were less likely to have AFO (Table 3). As the number of comorbidities increased, the RR of AFO decreased (one comorbidity, 0.87 [95% CI, 0.82-0.92]; two comorbidities, 0.75 [95% CI, 0.70-0.81]; three comorbidities, 0.58 [95% CI, 0.51-0.66]; four comorbidities, 0.51 [95% CI, 0.39-0.66]; five comorbidities, 0.47 [95% CI, 0.25-0.90]) (Table 3). All analyses were repeated using the GOLD definition of AFO, and results were similar (data not shown).
TABLE 3 ] .
RR of Airflow Obstruction on Spirometry Among Patients Given an ICD-9 Diagnosis of COPD on Empirical Treatment by Number of Total Comorbidities (N = 3,209)
| Comorbidity | RR (95% CI) | P Value |
| Total comorbidities | 0.85 (0.82-0.87) | < .001 |
| By number of comorbidities | ||
| 1 (n = 1,239) | 0.87 (0.82-0.92) | < .001 |
| 2 (n = 733) | 0.75 (0.70-0.81) | < .001 |
| 3 (n = 340) | 0.58 (0.51-0.66) | < .001 |
| 4 (n = 95) | 0.51 (0.39-0.66) | < .001 |
| 5 (n = 17) | 0.47 (0.25-0.90) | .02 |
| 6 (n = 1) | … | … |
Sensitivity Analyses
When the analysis was restricted to patients who received more-intensive inhaled medication treatment for COPD (six or more albuterol canisters, three or more ipratropium canisters, any LABA, or any ICS in the prior year), results were unchanged overall (Table 4). When the cohort was restricted to patients with prebronchodilator and postbronchodilator FEV1/FVC ratios, results were again unchanged overall (Table 5).
TABLE 4 ] .
Sensitivity Analysis: RR of Airflow Obstruction on Spirometry Among Patients Given an ICD-9 Diagnosis of COPD on More-Intense Treatment by Demographics and Comorbidities (n = 2,441)
| Covariatea | RR (95% CI) | P Value |
| White race | 0.96 (0.90-1.02) | .21 |
| Smoking in prior year | 1.05 (0.99-1.11) | .10 |
| Site of care | ||
| Rural clinical center | Reference | … |
| Tertiary center | 0.87 (0.82-0.93) | < .001 |
| Rural academic center | 1.13 (1.05-1.21) | .001 |
| Age | 1.002 (0.999-1.005) | .18 |
| BMI | ||
| Normal weight | Reference | … |
| Underweight | 1.18 (1.09-1.27) | < .001 |
| Overweight | 0.93 (0.87-0.98) | .01 |
| Obese | 0.75 (0.69-0.81) | < .001 |
| ACS | 0.99 (0.81-1.20) | .88 |
| CHF | 0.86 (0.78-0.94) | .001 |
| Depression | 0.91 (0.85-0.98) | .01 |
| Diabetes | 0.84 (0.77-0.91) | < .001 |
| Sleep apnea | 0.79 (0.68-0.92) | .003 |
| HTN | 0.92 (0.88-0.97) | .003 |
| Prior exacerbationsb | 1.05 (1.03-1.06) | < .001 |
More-intense treatment is defined as prescription of six or more short-acting β-agonist canisters, three or more ipratropium canisters, one or more long-acting β-agonists, or one or more inhaled corticosteroids in the prior year. See Table 1 and 2 legends for expansion of abbreviations.
All covariates were included in a single model.
Number of COPD exacerbations in the 2 y prior to the index date.
TABLE 5 ] .
Sensitivity Analysis: RR of Airflow Obstruction on Spirometry Among Patients Given an ICD-9 Diagnosis of COPD on Empirical Treatment With Postbronchodilator Spirometry (n = 2,813)
| Covariate | RR (95% CI) | P Value |
| White race | 0.95 (0.90-1.0) | .12 |
| Smoking in prior year | 1.07 (1.004-1.12) | .04 |
| Site of care | ||
| Tertiary centera | 0.91 (0.86-0.97) | < .01 |
| Rural academic centera | 1.32 (0.59-0.92) | < .01 |
| Age | 1.002 (0.999-1.006) | .12 |
| BMI | ||
| Normal weight | Reference | … |
| Underweight | 1.11 (1.02-1.22) | .02 |
| Overweight | 0.89 (0.84-0.94) | < .001 |
| Obese | 0.72 (0.67-0.77) | < .001 |
| ACS | 1.04 (0.87-1.24) | .70 |
| CHF | 0.84 (0.77-0.93) | < .001 |
| Depression | 0.91 (0.85-0.98) | < .01 |
| Diabetes | 0.89 (0.82-0.97) | < .01 |
| Sleep apnea | 0.86 (0.74-0.98) | .03 |
| HTN | 0.90 (0.86-0.95) | < .001 |
| Prior exacerbationsb | 1.07 (1.05-1.08) | < .001 |
Discussion
We found that a little over one-half of patients who receive a clinical diagnosis of and were treated for COPD have AFO on spirometry, raising the concern that patients are being inappropriately treated for a condition that they do not have. The current study is consistent with previous studies that suggested providers diagnose COPD and make treatment decisions based on history and clinical examination despite guideline recommendations to diagnose COPD with spirometry.5,6 Among veterans being treated for COPD, we identified common comorbidities such as obesity and CHF as factors associated with a lower RR of AFO. These conditions are common in the general population and often are associated with dyspnea.17,18 Treating patients who do not have AFO with inhaled medications may not only result in delays in diagnosis and treatment of the true etiology of dyspnea but also expose patients to the risk of adverse medication effects.12,13,19,20 We suggest that this care is likely ineffective, inefficient, and not patient centered.21
Many providers rely on symptoms to diagnose COPD, even though multiple studies have shown that diagnosis based on symptoms and physical examination is unreliable.7,22‐24 Previous studies have highlighted that only approximately one-third of patients given a new diagnosis of COPD undergo spirometry to verify the diagnosis.2,6 Studies in primary care physicians suggest that many providers believe that spirometry is not necessary to diagnose COPD.25‐27 Providers in these studies reported skepticism about the scientific evidence to perform spirometry and found interpretation of spirometry and guidelines confusing. Physicians also tended to prioritize COPD lower than other comorbidities during a routine clinic visit partly because they believed that diseases such as diabetes and HTN had more objective measures of disease status, whereas assessment of COPD is largely symptom based and subjective.25 The current study supports the idea that the diagnosis of COPD needs spirometric confirmation of AFO.
When COPD is diagnosed without spirometry, a patient’s dyspnea may be incorrectly attributed to nonexistent AFO. Dyspnea is the most common symptom among patients with COPD but is also common in other comorbidities such as CHF, depression, and obesity.17,18,28 The current finding that patients who have common comorbidities are less likely to have AFO on spirometry raises the concern that symptoms used to diagnose and guide treatment for COPD are actually due to other etiologies that may be underappreciated. This study supports prior evidence that patients with a greater number of comorbidities are less likely to have AFO on subsequent spirometry, suggesting that a symptom-based approach may be even less reliable among patients with multiple comorbidities.29
Similar to other studies, we demonstrated that older patients given an empirical diagnosis of COPD are more likely to have AFO.6,29‐31 We also found that predictors expected to increase the risk of AFO, such as tobacco consumption and being underweight, were associated with a higher risk of having AFO.32,33 Prior studies have shown that patients with more severe COPD (based on FEV1) were less likely to receive an inaccurate COPD diagnosis.34 We similarly expected prior COPD exacerbations (a measure of COPD severity) to be predictive of having AFO; however, the association was surprisingly weak, suggesting that patients may be treated for COPD exacerbations when they do not have COPD.
Treatment of patients without COPD with inhaled medications is an inefficient use of health-care resources and is neither cost-effective nor patient centered. COPD alone is associated with an increased use of health-care resources and higher health-care costs.35,36 Prior evidence suggests that patients considered false positive for COPD (a clinical diagnosis lacking AFO on spirometry) are more likely to be hospitalized and undergo more diagnostic tests than those considered true positive, potentially representing a missed opportunity for intervention in high-risk patients.3,37 Some patients in the present cohort taking inhaled medications may have had emphysema, simple chronic bronchitis, or air trapping in the absence of AFO. The benefit of inhaled medications to patients without AFO but with evidence of these other conditions alone is not well described and is not recommended by current guidelines.38‐40 Furthermore, inhaled medications used to treat COPD are costly and have been associated with adverse effects such as increased risk of pneumonia with ICS and possible adverse cardiovascular effects with inhaled anticholinergic medications or LABAs.11,13,19 This is of particular concern because patients at greater risk for receiving a diagnosis of and treatment for COPD in the absence of AFO are those who may also be at greater risk for adverse outcomes.
Although the current findings may seem to run counter to the well-characterized increase in prevalence of cardiac comorbidity among patients with COPD, the study explicitly examined patients who were given a diagnosis of and treated for COPD, raising the possibility that symptoms common to both were incorrectly attributed to COPD.41,42 The present findings highlight the importance of spirometry in COPD diagnosis, especially in medically complex patients.
This study had several limitations. First, the cohort largely comprised older male veterans, which may limit generalizability. Second, we could not identify whether patients received additional care or diagnoses outside the VA health-care system, leading to incomplete assessment of comorbid illnesses and treatment. We also do not know whether patients had prior spirometry. However, given the low prevalence of spirometry testing, we believe it unlikely that the patients had prior spirometry. Finally, we did not have data on patient symptoms and were unable to assess how symptoms may have correlated with the presence of AFO and with inhaled treatment. Despite these limitations, the study had a number of strengths, including a large sample of patients across various clinical sites representing an entire geographic region of the United States. In addition, we were able to capture not only spirometric results for all patients during this time period but also treatment and diagnoses in a complete and unbiased fashion.
Conclusions
Empirical diagnosis of COPD often is inaccurate, yet many patients who lack AFO are treated with inhaled medications. Patients with more comorbid illnesses are at greater risk for empirical diagnosis and treatment of COPD in the absence of AFO. Accurate diagnosis and treatment of COPD are important to improve quality of care, patient-centered outcomes, and resource utilization. Implementation of performance-based measures for COPD may improve the accuracy of diagnosis and treatment of patients suspected of having COPD.
Acknowledgments
Author contributions: B. F. C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. B. F. C. contributed to the study design, data analysis and interpretation, and preparation, review, and approval of the manuscript; L. C. F. and D. H. A. contributed to the design and conduct of the study, data collection, management, analysis, and interpretation, and preparation, review, and approval of the manuscript; and S. T. R. contributed to the review and approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Au has received funding from Gilead for work that is unrelated to this article and was an unpaid research consultant for Analysis Group. Drs Collins, Feemster, and Rinne have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding bodies played no role in the design of the study, analysis or interpretation of the findings, or drafting of the manuscript and did not review or approve the manuscript prior to submission.
Other contributions: The authors assume full responsibility for the accuracy and completeness of the findings presented. The views are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the National Institutes of Health.
ABBREVIATIONS
- AFO
airflow obstruction
- CHF
congestive heart failure
- HTN
hypertension
- ICD-9
International Classification of Diseases, Ninth Revision
- ICS
inhaled corticosteroid
- LABA
long-acting β-agonist
- RR
relative risk
- VA
US Department of Veterans Affairs
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 284
FUNDING/SUPPORT: This study was funded by an American Lung Association Career Investigator Award [CI-51755N]. Drs Collins and Rinne are supported by National Institutes of Health (NIH) [Training Grant T32 HL007287]. Dr Feemster is funded by an NIH National Heart, Lung, and Blood Institute K23 Mentored Career Development Award [K23 HL111116] and by the Department of Veterans Affairs (VA) Health Services Research and Development (HSR&D). Dr Au is supported by the VA HSR&D.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Endorsement summary: pulmonary and critical care measures. National Quality Forum website. https://www.qualityforum.org/News_And_Resources/Endorsement_Summaries/Endorsement_Summaries.aspx. Published 2012. Accessed May 24, 2013.
- 2.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403-409. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Agency for Healthcare Research and Quality website. http://www.qualitymeasures.ahrq.gov. Accessed May 24, 2013. [DOI] [PubMed]
- 4.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. [DOI] [PubMed] [Google Scholar]
- 5.Joish VN, Brady E, Stockdale W, Brixner DI, Dirani R. Evaluating diagnosis and treatment patterns of COPD in primary care. Treat Respir Med. 2006;5(4):283-293. [DOI] [PubMed] [Google Scholar]
- 6.Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129(6):1509-1515. [DOI] [PubMed] [Google Scholar]
- 7.Joo MJ, Au DH, Fitzgibbon ML, McKell J, Lee TA. Determinants of spirometry use and accuracy of COPD diagnosis in primary care. J Gen Intern Med. 2011;26(11):1272-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006;28(5):945-952. [DOI] [PubMed] [Google Scholar]
- 9.Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43(1):75-80. [DOI] [PubMed] [Google Scholar]
- 10.Bolton CE, Ionescu AA, Edwards PH, Faulkner TA, Edwards SM, Shale DJ. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005;99(4):493-500. [DOI] [PubMed] [Google Scholar]
- 11.Joo MJ, Au DH, Fitzgibbon ML, Lee TA. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med. 2010;104(2):246-252. [DOI] [PubMed] [Google Scholar]
- 12.Lee TA, Pickard AS, Au DH, Bartle B, Weiss KB. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149(6):380-390. [DOI] [PubMed] [Google Scholar]
- 13.Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175-1185. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation Geneva, Switzerland: World Health Organization; 2000. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 16.StataCorp. Stata Statistical Software. Release 120 [software] College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 17.Friedman MM. Older adults’ symptoms and their duration before hospitalization for heart failure. Heart Lung. 1997;26(3):169-176. [DOI] [PubMed] [Google Scholar]
- 18.Parshall MB. Adult emergency visits for chronic cardiorespiratory disease: does dyspnea matter? Nurs Res. 1999;48(2):62-70. [DOI] [PubMed] [Google Scholar]
- 19.Ogale SS, Lee TA, Au DH, Boudreau DM, Sullivan SD. Cardiovascular events associated with ipratropium bromide in COPD. Chest. 2010;137(1):13-19. [DOI] [PubMed] [Google Scholar]
- 20.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159(9):941-955. [DOI] [PubMed] [Google Scholar]
- 21.Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. [DOI] [PubMed] [Google Scholar]
- 22.Walters JA, Hansen E, Mudge P, Johns DP, Walters EH, Wood-Baker R. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34(3):201-203. [PubMed] [Google Scholar]
- 23.Holleman DR, Jr, Simel DL. Does the clinical examination predict airflow limitation? JAMA. 1995;273(4):313-319. [PubMed] [Google Scholar]
- 24.Buffels J, Degryse J, Heyrman J, Decramer M; DIDASCO Study. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO Study. Chest. 2004;125(4):1394-1399. [DOI] [PubMed] [Google Scholar]
- 25.Joo MJ, Sharp LK, Au DH, Lee TA, Fitzgibbon ML. Use of spirometry in the diagnosis of COPD: a qualitative study in primary care. COPD. 2013;10(4):444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salinas GD, Williamson JC, Kalhan R, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis. 2011;2011(6):171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3(2):311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162(13):1477-1481. [DOI] [PubMed] [Google Scholar]
- 29.Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust. 2011;195(4):168-171. [DOI] [PubMed] [Google Scholar]
- 30.Walters JA, Walters EH, Nelson M, et al. Factors associated with misdiagnosis of COPD in primary care. Prim Care Respir J. 2011;20(4):396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arne M, Lisspers K, Ställberg B, et al. How often is diagnosis of COPD confirmed with spirometry? Respir Med. 2010;104(4):550-556. [DOI] [PubMed] [Google Scholar]
- 32.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856-1861. [DOI] [PubMed] [Google Scholar]
- 33.Meyer PA, Mannino DM, Redd SC, Olson DR. Characteristics of adults dying with COPD. Chest. 2002;122(6):2003-2008. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg A, Bjerg A, Rönmark E, Larsson LG, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking: report from the Obstructive Lung Disease in Northern Sweden Studies [published correction appears in Respir Med. 2007;101(12):2569]. Respir Med. 2006;100(2):264-272. [DOI] [PubMed] [Google Scholar]
- 35.Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med. 2000;160(17):2653-2658. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2_suppl):5S-9S. [DOI] [PubMed] [Google Scholar]
- 37.Bunyan A, Lee TA, Au DH, Joo MJ. Healthcare utilization associated with misdiagnosis of COPD in primary care [abstract]. Am J Respir Crit Care Med. 2013;187:A5020. [Google Scholar]
- 38.Martinez CH, Kim V, Chen Y, et al. ; COPDGene Investigators. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108(3):491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease website. http://www.goldcopd.org. Published 2013. Accessed May 5, 2014.
- 41.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962-969. [DOI] [PubMed] [Google Scholar]
- 42.Müllerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163-1178. [DOI] [PubMed] [Google Scholar]