INTRODUCTION
Obesity has reached epidemic proportions in our society, affecting over one-third of US adults, with two-thirds overweight or obese (1). Trends toward overweight and obesity among younger age groups are alarming; 27.5% of men and 34.0% of women ages 20–39 are obese (1), and 11.3% of children 2–19 years of age are at or above the 97th percentile for 2000 BMI-for-age growth charts (2). The majority of overweight or obese individuals are also insulin resistant (3). The adverse health consequences of obesity and insulin resistance (IR) are well-documented, particularly with respect to cardiovascular disease and type 2 diabetes mellitus (T2DM). More recently, these conditions have also been linked to an increased risk of cognitive impairment and Alzheimer’s disease (AD) (4). AD is the most common cause of dementia, and the fifth leading cause of death in the United States among those 65 and older (5). The number of patients affected by AD in the United States is projected to increase from 5.3 million currently, to 16 million in 2050 as the population ages (5), imposing extraordinary monetary and non-monetary costs on patients, caregivers, and the healthcare system. Currently approved therapies for AD provide modest symptomatic benefits to some patients, but do not affect the underlying pathology. Identification of modifiable risk factors to delay or prevent progression to clinical dementia and functional impairment could have a dramatic impact on the prevalence and costs associated with AD. Although there is currently insufficient evidence to firmly link any modifiable risk factor with AD, substantial empirical evidence supports a role for several cardiovascular risk factors, including obesity, hypertension, dyslipidemia, diabetes, and IR (6). All of these factors have been implicated in the development and progression of AD, both individually and in aggregate (i.e., the metabolic syndrome) (7,8). A growing body of literature has demonstrated insulin dysregulation as a risk factor for both AD and its prodrome, mild cognitive impairment (4,9,10). Furthermore, IR represents a preclinical stage on the path to diabetes during which efforts at intervention are likely to have maximal effect. We focus here on the potential role of IR in the pathogenesis of AD, and discuss interventions that target IR as possible approaches to prevent or delay progression of AD.
DIABETES MELLITUS AND AD
Analysis of multiple longitudinal studies indicates that diabetes confers a 1.2- to 1.5-fold increased risk of cognitive decline, and a 1.6-fold increased risk of developing dementia (11). Studies specifically assessing the risk of AD associated with diabetes, adjusting for cardiovascular risk factors such as stroke, hypertension, heart disease, and cerebrovascular disease, have also demonstrated an increased risk; six of seven longitudinal population-based studies reviewed found an excess risk for AD in adults with diabetes ranging from 50–100% (12). Most studies do not differentiate between type 1 and T2DM, although T2DM is likely to predominate, especially in studies of older adults. Although the magnitude of the association is modest, the high prevalence of diabetes, particularly T2DM, translates into a potentially large impact on the number of AD cases. Furthermore, cognitive impairment may occur at an earlier age in persons with diabetes. Cigolle et al. recently reported increased prevalence and incidence of cognitive impairment in middle-aged adults with diabetes compared to those without (13). However, autopsy studies of AD in persons with diabetes have been inconsistent, with some (14), but not all (15,16), reporting an association between AD neuropathology and diabetes.
OBESITY, PERIPHERAL INSULIN, AND AD
The precursors of T2DM, obesity, IR, and hyperinsulinemia have been linked to an increased risk of cognitive impairment and AD (17–19). To date, the role of obesity in cognitive decline remains unclear. Studies have shown no association (20), an inverse association (21), or a U-shaped association (22), with both high and low BMI related to an increased risk of AD. A recent meta-analysis reported an increased risk (pooled effect size 1.59 (95% confidence interval 1.02–2.5; P < 0.05)) of AD risk with obesity (BMI ≥30 kg/m2) (4). However, the authors of this meta-analysis noted significant heterogeneity in the effect sizes, as well as a lack of information on the extent, distribution, and duration of obesity reported among studies, making it difficult to draw firm conclusions about the impact of obesity per se.
There is some evidence that an “ obesity paradox” akin to that described for cardiovascular disease may also exist for dementia (23). Mid-life obesity, assessed by both BMI and skin-fold thickness, has consistently shown a strong and independent association with an increased risk of dementia and AD (24). Studies of late-life obesity, however, have been mixed, with several well-conducted investigations demonstrating an inverse relation between late-life BMI and dementia. Analysis of subjects ≥65 years of age from the Cardiovascular Health Study found a 60% increased risk of incident dementia in those with BMI <20 kg/m2, and a 40% decreased risk in those with BMI >30 kg/m2, relative to normal weight subjects (25). Luchsinger et al. also reported a decreased risk of dementia with increasing BMI in subjects ≥76 years of age, but a U-shaped association in subjects <76 years of age (26).
It has been suggested that changes in body composition with age make BMI a poor measure of obesity in older subjects (24). In addition, weight loss may be part of the preclinical phase of AD, occurring before any detectable cognitive impairment (24). Consistent with this, the longitudinal PAQUID (Personnes Agées Quid) Study reported that while subjects with BMI <21 kg/m2 had an increased risk of dementia over 8 years of follow-up, exclusion of subjects who developed dementia before year 3 resulted in loss of significance (21).
Current understanding of the course of AD is that the pathology develops and progresses over many years before reaching a critical threshold at which symptoms appear. Thus, the presence of risk factors such as obesity and IR in mid-life may be far more important than they are in later life, when much of the damage has already occurred.
It is possible that peripheral insulin sensitivity mediates the effects of obesity on AD risk, contributing to the variability among studies, but IR has not been measured directly. Rather, most studies have simply looked at the surrogate, hyperinsulinemia. One study that performed euglycemic clamps in 1,125 older men found no association between IR and the development of AD, although a low early insulin response measured by oral glucose tolerance test did appear to increase the risk of AD (27). Nevertheless, insulin concentrations appear consistently higher in AD patients compared to controls (17,18,28,29). Taken together, the data suggest obesity and hyperinsulinemia are common in AD, but whether adiposity and peripheral insulin sensitivity mediate incidence of AD remains unknown. There is at least some evidence to suggest peripheral insulin sensitivity may impact the brain. In cognitively normal adults, IR, estimated by the homeostasis model assessment score (HOMA), was associated with a pattern of reduced cerebral glucose metabolism that has been shown to occur early in AD and to be predictive of the development of clinical AD (30). Higher levels of HOMA-IR and hyperinsulinemia have also been linked to an increased burden of amyloid plaques at autopsy over 10 years later (31).
CENTRAL INSULIN AND AD
Abnormalities in the concentration of insulin and insulin receptor, and in downstream signaling mechanisms in the brain have been reported in AD, leading some to propose that AD be considered “type 3 diabetes”, sharing features of type 1 and T2DM, but selectively affecting the brain (32). Reduced brain glucose metabolism has been well-described in AD, and documented very early in the disease process (33,34). AD subjects have also been shown to have both lower cerebrospinal fluid (CSF) insulin concentration and lower CSF:plasma insulin ratios than healthy controls (35). Postmortem studies have demonstrated striking reductions in central nervous system (CNS) expression of genes encoding both insulin and insulin receptor in AD cases compared to aged controls (36). Direct administration of insulin to the brain through the intranasal route (bypassing the blood–brain barrier) improved hippocampus-dependent memory function in subjects with early AD or mild cognitive impairment (37,38), suggesting that low-insulin concentrations in the brain may contribute to cognitive impairment.
Although AD appears to be associated with reduced insulin concentrations centrally, peripheral IR is typically characterized by increasing insulin concentrations with time. This apparent paradox may be explained in part by the finding that chronic peripheral hyperinsulinemia results in reduced transport of insulin across the blood– brain barrier, creating a state of central insulin deficiency (39). In humans, Kern et al. reported a decrease in the CSF:plasma insulin ratio with increasing IR (estimated by HOMA) (40). In dogs, adiposity induced by high-fat feeding was associated with reduced CNS insulin uptake, and a 60% reduction in CNS insulin concentrations (41).
In addition to reduced transport of insulin to the CNS, peripheral IR may induce a state of central IR, such that the brain becomes progressively less responsive to what little insulin is present. Reductions in stimulated and spontaneous cerebrocortical activity have been correlated with the degree of peripheral IR in obese subjects during hyperinsulinemic–euglycemic clamp studies (42). Evidence of central IR in persons with peripheral IR has also been reported using 18-fluorodeoxyglucose and positron emission tomography (FDG-PET) to measure cerebral glucose metabolism (43). Compared to insulin-sensitive subjects, IR subjects (defined by HOMA ≥2.77; mean 6.3) demonstrated significantly smaller increases in whole brain and regional glucose metabolism in response to insulin infusion. Although these studies do not establish causality, peripheral and central IR appear to coexist.
Whether deficient brain insulin concentrations and signaling are correlated with pathologic features of AD has not been well-studied in humans. In mice given a single intracerebral injection of streptozotocin to induce central IR (while maintaining normal peripheral glucose and insulin concentrations), brain expression of genes encoding insulin, insulin receptor, and the downstream-signaling molecule, insulin receptor substrate-1, was reduced compared to controls (32). These observations along with demonstration of reduced ligand binding to the insulin receptor strongly suggest impairment of insulin-signaling pathways in the treated animals. Streptozotocin-treated animals also displayed several classical features of AD including elevated amyloid precursor protein (APP), β-amyloid (Aβ) and phosphorylated tau protein, neuronal cell loss, diminished acetylcholine, and cerebral atrophy. Streptozotocin-treated animals had marked defects in learning and memory, compared to controls. These data suggest that central IR may affect the development of AD independent of peripheral IR. Thus, while peripheral IR may contribute to central IR by reducing the transport of insulin into the CNS, leading to a state of central insulin deficiency and impaired signaling, peripheral IR may not be a prerequisite of central IR. Rather, peripheral IR may function as a cofactor that contributes to, but does not cause AD, an idea that may help explain some of the inconsistencies in studies of obesity, IR, diabetes, and AD in humans to date.
INSULIN IN THE CNS
Insulin receptors are present throughout, but selectively distributed within the CNS. In rats, the highest concentrations of insulin receptors are found in the olfactory bulb, hypothalamus, cerebral cortex, cerebellum, and hippocampus (44). In humans, Steen et al. reported that levels of messenger RNA transcripts for the insulin receptor were dramatically higher in the hippocampus and hypothalamus compared to the frontal cortex (36). The distribution of insulin receptors in the brain overlaps with that of known downstream effectors of insulin signaling (45), and correlates well with insulin uptake (46). These observations suggest that insulin may have regional effects supporting normal functions in multiple areas of the brain, including those areas affected early in the course of AD (i.e., the hippocampus).
The physiologic functions of insulin in the brain are diverse and appear to be regionally specific. Examination of these functions provides insight into multiple mechanisms by which disruptions in brain insulin signaling may contribute to the development and progression of AD.
Although it was long believed that glucose uptake in the brain was completely independent of insulin, recent work has challenged that assumption. Bingham et al. used FDG-PET to study the effects of basal insulin administration on brain glucose metabolism (47). The authors reported increased brain glucose uptake in response to insulin in the cortex, but not the cerebellum or brainstem. In support of this finding, partially insulin-sensitive glucose transporters have been identified in the CNS. Although definitive studies have not been done to date, research thus far supports the possibility that selected areas of the brain exhibit insulin-dependent glucose uptake. As noted above, abnormalities of cerebral glucose metabolism have been well-described in AD. A pattern of reduced glucose metabolism in the parietal–temporal, posterior cingulate, and prefrontal association cortices with relative sparing of the cerebellum, thalamus, and basal ganglia has been consistently demonstrated in FDG-PET studies of AD patients, with an estimated 90% sensitivity for identifying AD (34). Metabolic imaging of the hippocampus, which is particularly vulnerable to damage in neuropathologic studies of AD, is challenging, but several studies combining magnetic resonance imaging with PET for more detailed sampling have demonstrated hippocampal glucose hypometabolism in both AD and mild cognitive impairment (34). Whether the areas of reduced brain glucose metabolism observed in AD correlate with those areas of the brain that show evidence of insulin-sensitive glucose uptake is not known. Further study of the possible relation between CNS insulin dysregulation and the cerebral hypometabolism characteristic of AD is needed.
Insulin has also been shown to support normal cognitive function in both animals and humans. Intracerebroventricular (48) and intrahippocampal (49) injections of insulin improved performance on a passive-avoidance task in rats that reflects enhanced memory for negative consequences. Infusion of intravenous insulin, which raises CNS insulin, also improved memory in healthy human adults (50). Likewise, administration of intranasal insulin directly to the CNS has been shown to improve memory in cognitively normal adults (51).
The molecular mechanisms for the role of insulin in learning and memory are not well-understood, but may be distinct from effects on glucose metabolism (52). Insulin is known to interact with several neurotransmitter systems important in synaptic plasticity, the ability of neurons to change the structure and strength of their connections in response to various stimuli. Synaptic plasticity is regarded as a key neurochemical foundation underlying new learning and memory. Insulin recruits N-methyl-D-aspartic acid (NMDA) receptors to the plasma membrane, significantly potentiating receptor activity (52). Calcium flux through NMDA receptors subsequent to activation by coligands glutamate and glycine is thought to be critical in facilitating synaptic plasticity. Insulin also modulates transmission of γ-aminobutyric acid (GABA), a key mediator of synaptic inhibition associated with learning (52). Moreover, several signaling pathways downstream of insulin receptor activation, including Shc/mitogen-activated protein (MAP) kinase, phospholipase C (PLC)/protein kinase C (PKC), and phosphatidylinositol-3 (PI-3) kinase, have been linked to the transcription of genes involved in memory formation and consolidation (52). Finally, insulin stimulates the production of the potent vasodilator nitric oxide (NO) in vascular endothelial cells through activation of endothelial NO synthase (eNOS). eNOS is expressed in hippocampal neurons, and has been implicated as a messenger molecule in long-term potentiation, a form of synaptic plasticity in which repeated stimulation of neuronal presynaptic terminals augments synaptic transmission over a period of hours to days (53). In rats, intrahippocampal administration of the eNOS inhibitor N-nitro-L-arginine methyl ester attenuated insulin-induced memory enhancement (54).
INSULIN AND AD PATHOLOGY
Insulin, insulin receptors, and impairments in insulin signaling have been implicated in the neuropathology of AD, namely, the amyloid plaques and neurofibrillary tangles (NFT) that characterize the disease. The amyloid hypothesis has been a central, albeit controversial, theory of AD pathogenesis since it was proposed in the early 1990s (55). According to the amyloid hypothesis, it is the accumulation and aggregation of Aβ peptide that initiates and perpetuates AD neurodegeneration. Aβ is formed from cleavage of amyloid precursor protein by β- and γ-secretases and is prone to aggregate into toxic oligomers (56). Over time, these oligomers may merge to form insoluble amyloid fibrils, and eventually, the plaques that have come to characterize AD.
Studies in neuronal cell cultures have demonstrated that activation of insulin receptors facilitated the reduction of toxic Aβ oligomers to less noxious monomers, preventing synaptic toxicity, a process that may be mediated by insulin-degrading enzyme (57). Both insulin and Aβ are substrates of this metalloprotease, which appears to be important in clearing Aβ from the brain. Conversely, transfection of cells with dysfunctional insulin receptors derived from human subjects with IR, was associated with increased accumulation of toxic Aβ oligomers (57). Aβ oligomers have also been shown to cause insulin receptor dysfunction (58), potentially leading to a deleterious bidirectional cycle. That is, defective insulin receptors or downstream signaling may facilitate accumulation of Aβ oligomers, which in turn further disrupt insulin signaling, perpetuating the neuropathology of AD.
NFT, consisting of aggregations of abnormally phosphorylated tau proteins, are the other neuropathologic hallmark of AD (56). Tau normally binds to microtubules in axons, promoting their assembly and stabilization. Pathological hyperphosphorylation of tau leads to self-aggregation, formation of paired helical filaments, and eventually NFT (56). This process destabilizes microtubules, impairing axonal transport, and resulting in neuronal dysfunction and degeneration (56). The tau hypothesis of AD posits that tau hyperphosphorylation is a final common pathway in AD resulting from multiple upstream impairments, including Aβ formation (59). Impaired insulin signaling has been proposed as one of the upstream impairments that may lead to tau hyperphosphorylation (59). One of the key phosphorylators of tau is glycogen synthase kinase-3 (GSK-3), a ubiquitously expressed, constitutively active serine/ threonine protein kinase that has been identified in NFT in the brains of AD patients (60). Overexpression of GSK-3 in mice results in tau hyperphosphorylation, although studies have not consistently demonstrated subsequent formation of NFT (60). Insulin inhibits GSK-3 through the PI-3-kinase pathway activating glycogen synthase (60). Conversely, active GSK-3 phosphorylates insulin receptor substrate-1, attenuating insulin signaling (60). GSK-3 therefore functions as a key mediator of insulin signaling, facilitating glycogen synthesis in the presence of insulin and attenuating signaling in its absence. Adipose tissue and skeletal muscle GSK-3 activity appear to be increased in obesity and diabetes. In obese, diabetic mice, adipose tissue GSK-3 activity was double that of controls (60). In humans with T2DM, skeletal muscle GSK-3 expression and activity were increased compared to healthy controls (60). If the increased GSK-3 in obesity and diabetes results in increased phosphorylation of tau and NFT formation, this may be one mechanism by which impairments in insulin signaling contribute to AD risk. Indeed, several GSK-3 inhibitors are currently being studied as potential AD therapies (60).
INSULIN, VASCULAR DYSFUNCTION, AND AD PATHOLOGY
It is increasingly acknowledged that vascular dysfunction is a major component of AD, and that AD and vascular dementia, once considered distinct entities, likely occupy a continuum, with most patients having features of both. IR, through its effects on both vascular and classical AD pathology, offers a potentially compelling mechanism to help bridge these two pathways of cognitive decline.
Obesity and IR are associated with endothelial dysfunction, which is defined as impaired vasodilator response, and is an independent predictor of cardiovascular events, detectable long before the clinical manifestations of overt cardiovascular disease (61). In obese individuals, there appears to be an imbalance between vasodilatory and vasoconstrictive forces that favors vasoconstriction, and it has been proposed that abnormalities of insulin action associated with obesity may be partly responsible for this imbalance (62). Insulin stimulates eNOS, a potent vasodilator, as well as endothelin (ET)-1, a potent vasoconstrictor, thus insulin may be important in maintaining a healthy balance between these opposing effects on the vasculature (62). “Selective IR” has been described, in which IR impairs the pathways involved in insulin’s actions on NO, but not on those for ET-1, tipping the balance in favor of vasoconstriction (63). In support of this view, enhanced ET-1 activity has been shown to contribute to endothelial dysfunction in IR subjects with obesity or T2DM (64–66). However, Lteif et al. failed to demonstrate augmentation of ET-1 production or action with hyperinsulinemia in obese vs. lean subjects (63), suggesting a more complex interaction between NO and ET-1 in IR states.
Whether the endothelial dysfunction characteristic of IR states extends to the intracranial vasculature is unknown. Diabetes is strongly associated with cerebrovascular disease (67), which frequently coexists with AD pathology (68). Cerebrovascular disease may lower the threshold for the degree of AD pathology needed to cause symptomatic disease, as less AD pathology has been observed in dementia patients with infarcts compared to those without infarcts (69,70). Moreover, diabetes may further worsen the cerebrovascular effects on AD pathology. The Adult Changes in Thought Study demonstrated greater Aβ plaque burden and free radical damage (measured by F2-isoprostanes in the cerebral cortex) in AD subjects without diabetes, but significantly more microvascular infarcts and neuroinflammation (measured by cortical interleukin-6 (IL-6)) in AD subjects with diabetes (71). The effects of obesity and IR on the vasculature and AD pathology may be mediated by elevated free fatty acids (FFA) and tumor necrosis factor (TNF)-α associated with these conditions (62). Both FFA and TNF-α increase production of ET-1 and reactive oxygen species which further impair vascular function (72). In addition, both FFA and TNF-α may play more direct roles in AD. Although the potential role of FFA in AD has not been well-studied, FFA do inhibit insulin-degrading enzyme, a key enzyme in Aβ clearance (39). In addition, FFA have been shown to stimulate Aβ and tau protein aggregation in vitro (39). TNF-α inhibits clearance of Aβ from the CNS, and elevated brain and CSF concentrations of TNF-α have been demonstrated in both AD and mild cognitive impairment. Craft and colleagues observed significant increases in multiple inflammatory markers in the CSF, including TNF-α, in healthy older adults during hyperinsulinemic–euglycemic clamp studies (73). Further, increases in TNF-α were correlated with BMI, suggesting that obesity may exacerbate the inflammatory effects of peripheral insulin in the brain.
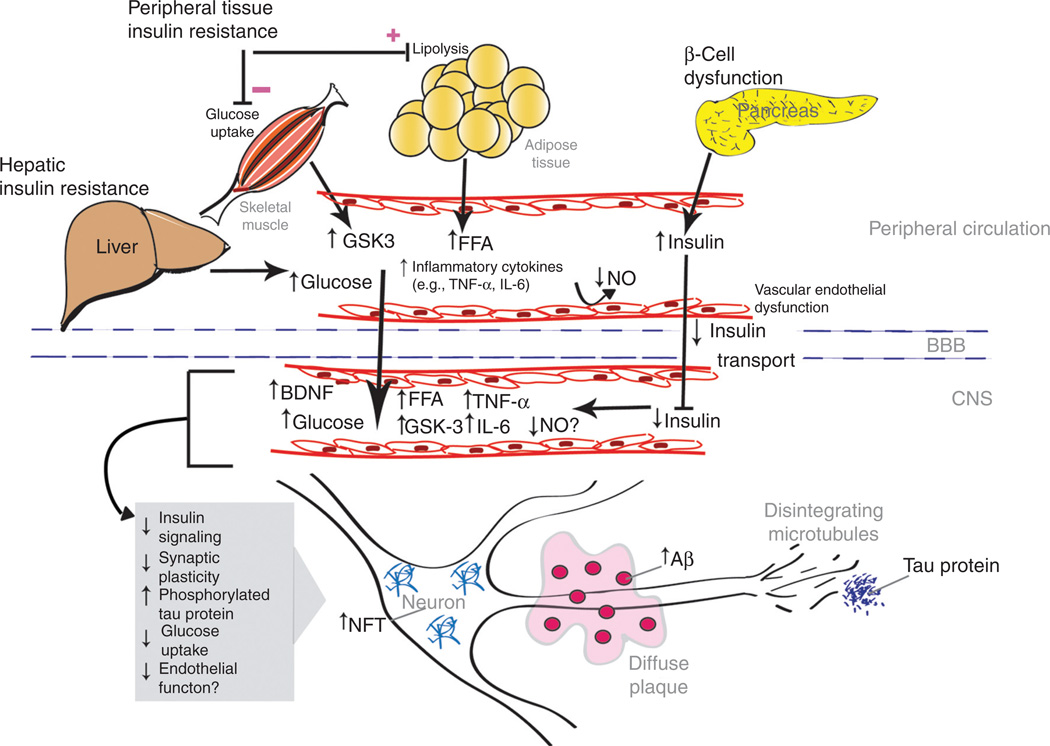
Much work remains to be done to completely elucidate the possible role of insulin in cognitive impairment and AD, and how peripheral insulin dysregulation may affect central insulin action. However, the evidence to date strongly supports such a role with multiple potential mechanisms by which insulin may contribute to the development and progression of cognitive decline and AD (Figure 1).
Figure 1.
Peripheral disorders on the spectrum of obesity, insulin resistance, and hyperinsulinemia may promote a state of central insulin deficiency and resistance that contributes to the development and progression of cognitive impairment and Alzheimer’s disease through multiple potential mechanisms. Prolonged peripheral hyperinsulinemia decreases insulin transport into the CNS, which may result in central insulin deficiency, resistance, and impaired signaling. These abnormalities may have diverse effects including decreased glucose metabolism and synaptic plasticity, as well as accumulation of Aβ and NFT that characterize Alzheimer’s disease. Decreased availability of NO may both impair synaptic plasticity and promote endothelial dysfunction. Known peripheral effects of obesity, insulin resistance and hyperinsulinemia, including endothelial dysfunction and elevations of FFA, TNF-α, and GSK-3, may extend to the CNS. FFA and TNF-α may promote intracranial endothelial dysfunction and stimulate accumulation of Aβ and NFT. Aβ, β-amyloid; BBB, blood–brain barrier; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; FFA, free fatty acids; GSK-3, glycogen synthase kinase-3; IL-6, interleukin-6; NFT, neurofibrillary tangles; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
POTENTIAL INSULIN-SENSITIZING THERAPEUTIC APPROACHES
The evidence linking IR to the development of AD is compelling, however, the critical question of whether treating IR can delay or prevent cognitive decline remains unanswered. There are both pharmacologic and nonpharmacologic treatments for IR, several of which have been studied for their effects on cognition.
Thiazolidinediones (TZDs) are synthetic agonists for the peroxisome proliferator-activated receptor-γ (PPAR-γ), a transcription factor highly expressed in adipose tissue, where it regulates the expression of genes involved in adipocyte differentiation, and carbohydrate and lipid metabolism (74). Initially studied as potential lipid-lowering agents, TZDs were found to decrease plasma insulin and glucose, and to improve insulin sensitivity. Two TZDs, rosiglitazone, and pioglitazone, are currently approved for the treatment of T2DM yet the mechanisms by which they improve insulin sensitivity are not completely understood. Through PPAR-γ, these drugs regulate the expression of over 100 different genes, thus, it is likely that multiple mechanisms are involved (74).
In addition to their effect on IR, TZDs have other actions that make them particularly interesting as potential therapies in preventing AD. TZDs have anti-inflammatory effects, and have been shown to decrease multiple markers of inflammation in humans (74). TZDs exert favorable effects on vascular function, enhancing vasodilation by increasing endothelial nitric oxide release, and reducing smooth muscle cell proliferation and migration (74). TZDs may also have a more direct role in the amyloid cascade; recent evidence suggests that PPAR-γ agonists may both repress expression of the β-secretase β-amyloid cleaving enzyme (BACE)-1, a key enzyme in the pathologic cleavage of amyloid precursor protein to Aβ, and may also stimulate clearance of existing Aβ (75). In animal studies, both rosiglitazone (76) and pioglitazone (77) have been shown to have favorable effects on AD pathology, including reductions in amyloid plaque burden, activated microglia, inflammatory markers, and Aβ levels in the brains of mice treated with TZDs.
Three studies of rosiglitazone in humans have been conducted. A pilot study of 30 subjects with mild AD or mild memory impairment showed better delayed recall and selective attention in subjects treated with rosiglitazone compared to placebo (78). Rosiglitazone treatment was also associated with stable, as opposed to declining, plasma Aβ, suggesting attenuated disease progression. A larger study of 511 patients with mild to moderate AD found a significant improvement in general cognitive function (as measured by the Alzheimer’s Disease Assessment Scale–Cognitive, ADAS-Cog), following treatment with rosiglitazone (79). However, a recent phase III trial of rosiglitazone, failed to replicate this finding, reporting no change in cognition with rosiglitazone relative to controls (80). Furthermore, concerns about the cardiovascular safety profile of rosiglitazone will likely discourage its use in future studies.
Pioglitazone has not been as widely studied; one small study in Japanese subjects with T2DM and either mild to moderate AD or mild cognitive impairment reported significant improvements in both memory and ADAS-Cog scores in subjects treated with pioglitazone compared to placebo (81). Geldmacher et al. recently reported that pioglitazone was well-tolerated in 29 nondiabetic subjects with AD over an 18-month period (82), but no effects were seen with pioglitazone on the secondary cognitive outcomes. Pioglitazone does have several potential advantages compared to rosiglitazone, including a favorable effect on lipid profiles, clear ability to cross the blood–brain barrier, and possibly less overall cardiovascular risk. Thus, pioglitazone may remain a viable target for development.
With regard to non-TZD insulin-sensitizing agents, a study of the biguanide metformin on APP metabolism and Aβ accumulation found that unlike TZDs, metformin increased Aβ accumulation by upregulating BACE-1 expression and activity (83), emphasizing the importance of the pleiotropic effects of insulin-sensitizing medications.
Endurance exercise has multiple beneficial metabolic effects (84–86), including improvement of IR, and may therefore represent another potential therapeutic target for preventing or delaying the progression of AD. Numerous studies have established that endurance exercise can improve IR (87). The acute effect of exercise on IR is through an effect on glucose transporter type-4 (GLUT-4) (87), the primary glucose transporter in skeletal muscle, whereas chronic effects of exercise may be mediated through reductions in central adiposity (88), inflammation (89), and vascular function (90).
Although no randomized controlled trial to date has shown that exercise can prevent or delay the onset of AD, there is strong epidemiological evidence that exercise may have beneficial effects on cognitive function. A review of 16 longitudinal epidemiologic studies found that 11 of the studies reported a significant association between physical activity and a reduced risk of dementia and/or AD (91). Such studies have many potential sources of bias, including questionable assessment of physical activity and inability to ascertain the pattern of physical activity over an individual’s lifetime. Results from randomized controlled trials designed to more rigorously test the effects of exercise on cognitive function have been mixed. A recent Cochrane review found that 8 of 11 exercise intervention randomized controlled trials in cognitively normal older adults reported an association between increased cardiorespiratory fitness and improvements in multiple aspects of cognitive function (92). Lautenschlager et al. examined the effects of an exercise intervention in 170 adults >50 years of age with subjective memory complaints, 60% of whom met formal criteria for mild cognitive impairment (93). In this population at high risk for developing AD, the authors reported that a very modest increase in physical activity (20 min more per day, mostly walking) improved general cognitive function (measured by the ADAS-Cog) relative to controls. Although modest, the improvements were comparable to those demonstrated with currently approved AD drug treatments.
Recent studies have provided further insight into potential mechanisms by which exercise may improve cognition. Baker et al. studied 28 cognitively normal older adults with abnormal glucose tolerance measured by 2-h oral glucose tolerance test, randomized to either 6-months of aerobic exercise or a stretching control group (94). In these subjects at increased risk of cognitive decline, the aerobic exercise group demonstrated significant improvements in cardiorespiratory fitness (measured by peak oxygen consumption), which were correlated with improvements in IR (measured by hyperinsulinemic–euglycemic clamp). The exercise group also showed significant improvement on tests of executive cognitive function, but not short-term memory, relative to the control group. This work supports previous findings of positive effects on executive function with exercise (91,92), and suggests improvements in insulin sensitivity may, at least in part, mediate these effects.
Exercise also appears to increase levels of serum brain-derived neurotrophic factor (BDNF), which has been reported to promote neurogenesis, dendritic expansion, and hippocampal synaptic plasticity underlying memory formation (95). BDNF has also been proposed as a key mediator of the interface between metabolism and synaptic plasticity. In diabetic mice, administration of BDNF has been shown to lower fasting and non-fasting blood glucose (96), and to enhance insulin-stimulated PI-3 kinase activation and peripheral glucose utilization (97). In humans, Arentoft et al. found lower plasma BDNF levels in older women with impaired insulin action (type 2 diabetes or IR) compared to controls; these subjects also performed more poorly on tests of memory compared to controls (98).
A recent trial in 120 cognitively normal older adults (ages 55–80) reported a 12-month aerobic exercise intervention selectively increased the volume of the anterior hippocampus relative to a stretching control group, and the increased volume was associated with increases in serum BDNF (99). Although changes in aerobic fitness (measured by maximal oxygen consumption, VO2max), and serum BDNF were not associated with improvements on a spatial memory task, modest correlations were found between increased hippocampal volume and spatial memory. These findings suggest that aerobic exercise may augment molecular pathways in selective brain regions important for memory and vulnerable to the effects of both normal aging and neurodegenerative diseases such as AD.
Exercise thus appears to have multiple effects that may positively affect cognitive function. Combined with the established benefits of exercise on multiple chronic illnesses (100) and the lack of side effects when properly performed, exercise presents an extremely attractive therapy to potentially prevent or delay cognitive decline and AD.
The complexity of AD implies that a multifaceted approach to prevention and treatment will likely be needed. Interventions that improve IR as one of their effects may have an important place in conjunction with other therapies targeting different or overlapping aspects of AD pathology.
CONCLUSIONS
Research conducted over the past 20 years clearly supports a role for insulin action that extends well beyond peripheral glucose metabolism. Insulin is active throughout the CNS in key pathways of learning and memory, and abnormalities in insulin action have increasingly been linked to neurodegenerative processes characteristic of AD and its prodrome, mild cognitive impairment. Taken together, these observations suggest that not only is insulin important for normal cognitive functioning, but that impaired insulin action and signaling may promote and/or exacerbate cognitive decline.
Unraveling the precise mechanisms by which IR may contribute to the development and progression of AD promises to be a rich area of continued investigation. Whether interventions that target IR can delay or prevent AD will be a critical question as we face an aging population that is increasingly overweight or obese. Moreover, with the earlier onset of obesity and IR, we may begin to observe cognitive decline at younger ages, further increasing the burden of AD. The limited data available to date suggest that both pharmacologic and nonpharmacologic interventions that improve IR may have beneficial effects on cognitive function. Well-designed, controlled intervention trials that assess cognitive outcomes and explore mechanisms in populations at high risk for developing AD are needed. The link between IR and AD affords the opportunity to address two of the most common problems in the elderly population, potentially improving the health and functional status of patients, decreasing caregiver burden, and benefiting the health care system and society as a whole.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health, National Institute on Aging, (AG019339, AG028746, AG000279), and the Hartford Center of Excellence in Geriatrics.
Footnotes
DISCLOSURE
The authors declared no conflict of interest. © 2012 The Obesity Society
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 4.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. 2010 Alzheimer’s Disease Facts and Figures. 2010 doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Rockville MD: Agency for Healthcare Research and Quality; 2010. Apr, Preventing Alzheimer’s disease and cognitive decline. Evidence Report/Technology Assessment No. 193. (Prepared by the Duke Evidence-based Practice Center under Contract No. HHSA 290-2007-10066-I) AHRQ Publication No. 10-E005. [Google Scholar]
- 7.Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67:843–847. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 9.Vanhanen M, Koivisto K, Kuusisto J, et al. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care. 1998;21:398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Blackwell T, Kanaya AM, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 11.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 12.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 13.Cigolle CT, Lee PG, Langa KM, et al. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med. 2011;26:272–279. doi: 10.1007/s11606-010-1510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 15.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 16.Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 17.Kuusisto J, Koivisto K, Mykkänen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 19.Schrijvers EM, Witteman JC, Sijbrands EJ, et al. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Nourhashémi F, Deschamps V, Larrieu S, et al. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorner TE, Rieder A. Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol. 2012;155:56–65. doi: 10.1016/j.ijcard.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 24.Whitmer RA. The epidemiology of adiposity and dementia. Curr Alzheimer Res. 2007;4:117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rönnemaa E, Zethelius B, Sundelöf J, et al. Glucose metabolism and the risk of Alzheimer’s disease and dementia: a population-based 12 year follow-up study in 71-year-old men. Diabetologia. 2009;52:1504–1510. doi: 10.1007/s00125-009-1393-9. [DOI] [PubMed] [Google Scholar]
- 28.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 29.Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer’s disease. Age Ageing. 1994;23:396–399. doi: 10.1093/ageing/23.5.396. [DOI] [PubMed] [Google Scholar]
- 30.Baker LD, Cross DJ, Minoshima S, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki T, Sasaki K, Tanizaki Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 32.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Leon MJ, Mosconi L, Blennow K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 34.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 35.Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 36.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease-is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 37.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 38.Reger MA, Watson GS, Frey WH, 2nd, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 40.Kern W, Benedict C, Schultes B, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006;49:2790–2792. doi: 10.1007/s00125-006-0409-y. [DOI] [PubMed] [Google Scholar]
- 41.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 42.Tschritter O, Preissl H, Hennige AM, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA. 2006;103:12103–12108. doi: 10.1073/pnas.0604404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anthony K, Reed LJ, Dunn JT, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 44.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 45.Hörsch D, Kahn CR. Region-specific mRNA expression of phosphatidylinositol 3-kinase regulatory isoforms in the central nervous system of C57BL/6J mice. J Comp Neurol. 1999;415:105–120. [PubMed] [Google Scholar]
- 46.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 47.Bingham EM, Hopkins D, Smith D, et al. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 48.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 49.Babri S, Badie HG, Khamenei S, Seyedlar MO. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn. 2007;64:86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 51.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 53.Huang EP. Synaptic plasticity: a role for nitric oxide in LTP. Curr Biol. 1997;7:R141–R143. doi: 10.1016/s0960-9822(97)70073-3. [DOI] [PubMed] [Google Scholar]
- 54.Choopani S, Moosavi M, Naghdi N. Involvement of nitric oxide in insulin induced memory improvement. Peptides. 2008;29:898–903. doi: 10.1016/j.peptides.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 56.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 57.Zhao WQ, Lacor PN, Chen H, et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta} J Biol Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 59.Maccioni RB, Farías G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch Med Res. 2010;41:226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med. 2002;8:126–132. doi: 10.1016/s1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- 61.Landmesser U, Drexler H. The clinical significance of endothelial dysfunction. Curr Opin Cardiol. 2005;20:547–551. doi: 10.1097/01.hco.0000179821.11071.79. [DOI] [PubMed] [Google Scholar]
- 62.Tesauro M, Cardillo C. Obesity, blood vessels and metabolic syndrome. Acta Physiol (Oxf) 2011;203:279–286. doi: 10.1111/j.1748-1716.2011.02290.x. [DOI] [PubMed] [Google Scholar]
- 63.Lteif AA, Fulford AD, Considine RV, et al. Hyperinsulinemia fails to augment ET-1 action in the skeletal muscle vascular bed in vivo in humans. Am J Physiol Endocrinol Metab. 2008;295:E1510–E1517. doi: 10.1152/ajpendo.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cardillo C, Campia U, Iantorno M, Panza JA. Enhanced vascular activity of endogenous endothelin-1 in obese hypertensive patients. Hypertension. 2004;43:36–40. doi: 10.1161/01.HYP.0000103868.45064.81. [DOI] [PubMed] [Google Scholar]
- 65.Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007;56:728–734. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- 66.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 67.Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- 68.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 69.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 70.Riekse RG, Leverenz JB, McCormick W, et al. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J Am Geriatr Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 73.Fishel MA, Watson GS, Montine TJ, et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62:1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- 74.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 75.Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer’s disease: therapeutic implications. CNS Drugs. 2008;22:1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen WA, McMillan PJ, Kulstad JJ, et al. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 77.Heneka MT, Sastre M, Dumitrescu-Ozimek L, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 78.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 79.Risner ME, Saunders AM, Altman JF, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 80.Gold M, Alderton C, Zvartau-Hind M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- 82.Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension: an update. Sports Med. 2000;30:193–206. doi: 10.2165/00007256-200030030-00004. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz RS, Cain KC, Shuman WP, et al. Effect of intensive endurance training on lipoprotein profiles in young and older men. Metab Clin Exp. 1992;41:649–654. doi: 10.1016/0026-0495(92)90058-i. [DOI] [PubMed] [Google Scholar]
- 86.Stewart KJ, Bacher AC, Turner K, et al. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med. 2005;28:9–18. doi: 10.1016/j.amepre.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 88.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 89.Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujita S, Rasmussen BB, Cadenas JG, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rolland Y, Abellan van Kan G, Vellas B. Healthy brain aging: role of exercise and physical activity. Clin Geriatr Med. 2010;26:75–87. doi: 10.1016/j.cger.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 93.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 94.Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22:569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 96.Tonra JR, Ono M, Liu X, et al. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588–594. doi: 10.2337/diabetes.48.3.588. [DOI] [PubMed] [Google Scholar]
- 97.Yamanaka M, Tsuchida A, Nakagawa T, et al. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes Metab. 2007;9:59–64. doi: 10.1111/j.1463-1326.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 98.Arentoft A, Sweat V, Starr V, et al. Plasma BDNF is reduced among middle-aged and elderly women with impaired insulin function: evidence of a compensatory mechanism. Brain Cogn. 2009;71:147–152. doi: 10.1016/j.bandc.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agency for Healthcare Research and Quality and the Centers for Disease Control. Physical activity and older Americans: benefits and strategies. 2002 Jun; < http://www.ahrq.gov/ppip/activity.htm>. [Google Scholar]