Abstract
OBJECTIVE
To find the nature of tendon involvement in chronic kidney disease (CKD) patients on regular hemodialysis (RD), and its relationship to parathyroid hormone (PTH) level using ultrasonography (US).
METHOD
A total of 50 CKD patients on RD subjected to musculoskeletal examination of knee and ankle, laboratory evaluation, and US of quadriceps tendon and Achilles tendon were involved.
RESULTS
Ankle joint tenderness was the most frequent sign on examination. US of the Achilles tendons showed tenderness during probing in 44% patients, calcific deposition in 24% patients, abnormal peritendon tissue in 20% patients, and abnormal anteroposterior (A-P) middle and distal one-third thicknesses of the Achilles tendon in 20% and 18% patients, respectively. PTH positively correlated with the duration of dialysis, serum phosphorus level, presence of calcific deposit, and increased thickness of the Achilles tendon.
CONCLUSION
The most common ultrasonographic finding in CKD patients on RD was Achilles tendon tenderness during probing. PTH level positively correlated with the duration of dialysis, presence of calcific deposit, and increased thickness of Achilles tendon.
Keywords: chronic kidney disease (CKD), musculoskeletal ultrasound, Achilles tendon, parathyroid hormone (PTH), renal osteodystrophy (ROD)
ACRONYMS: Chronic kidney disease (CKD), parathyroid hormone (PTH), anteroposterior (A-P), tuberculosis (TB)
Introduction
End-stage renal disease is associated with increased prevalence of physical inactivity, reduced exercise capacity, and different grades of disability.1 Implementation of physical activity both during and out of the dialysis session is warranted and useful to enhance quality of life and physical performance, and to reduce the risk of mortality and hospitalization.2
Renal osteodystrophy (ROD) has been described in patients with chronic kidney disease (CKD). Special importance has been given to secondary hyperparathyroidism, and osteomalacia and osteoporosis, dynamic bone diseases and soft-tissue or vascular calcifications. Less common musculoskeletal manifestations include aluminum, amyloid, and/or crystal deposition; destructive spondyloarthropathy; avascular necrosis; and tendon ruptures.3
In particular, spontaneous ruptures of the quadriceps tendon and/or the Achilles tendon in CKD patients with secondary hyperparathyroidism have been sporadically reported and are usually regarded as isolated events. Literature data regarding the etiology are controversial, and the predisposing factors behind such ruptures still remain unknown.4 Some authors speculate that the tendon remains intact, and the cause of rupture is an osteolytic bone resorption at the tendon insertion site, caused by secondary hyperparathyroidism and excess of parathyroid hormone (PTH).5
Although magnetic resonance imaging remains one of the main diagnostic imaging modalities for evaluating joint pathology worldwide, there are a number of useful applications and advantages of diagnostic ultrasound in the assessment of musculoskeletal pathology. Ultrasonography (US) is used to assess superficial tendons and ligaments that traverse a joint. It can demonstrate the presence and characteristics of joint effusions, bursae, or cysts, and it can also detect loose bodies in joints. The advantages lie in the cost-efficiency, the shorter examination time, and the ability for real-time and dynamic imaging. Its portability allows one to perform imaging of the anatomic structure in question and to perform rapid side-to-side comparisons.6
The current study aimed to investigate the frequency and nature of the Achilles tendon and quadriceps tendon affection as a sample of tendon involvement in patients with renal failure undergoing regular hemodialysis, and its relationship to PTH level, using ultrasonographic examination.
Patients and Methods
In a cross-sectional study, 50 CKD patients (27 females and 23 males) on regular hemodialysis for at least six months received a four-hour dialysis session thrice weekly; they were from a pool of patients from a dialysis unit in El-Sahel Teaching Hospital. Patients with systemic inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus as a cause of renal failure, previous trauma and bone fracture, or tuberculosis (TB) were excluded. The ethics committee of Ain Shams University reviewed and approved the patient consents for the study, and in accordance with the committee’s policies, no further ethics approval was required. The research was conducted in accordance with the principles of the Declaration of Helsinki. After informed written consent, all the patients subjected to full medical history [including etiology of CKD, duration of hemodialysis (in years), and musculoskeletal manifestations], musculoskeletal examination of knee and ankle, and laboratory evaluation [including complete blood count (CBC) done on Cell Dyn 1700; serum creatinine and blood urea nitrogen (BUN), serum calcium (Ca), and serum phosphorus (PO4) done on autoanalyzer BioLis 24I; and PTH by intact parathyroid hormone (iPTH) ELISA kits provided by GenWay Biotech Inc.]. This ELISA is a sandwich technique that uses polyclonal antibodies (P-Ab; goat anti 1–34 PTH fragment) coated on micro titer well and monoclonal antibodies (M-Ab; mouse anti 44–68 PTH fragment) labeled with horseradish peroxidase (HRP). Ultrasonographic evaluation was performed by an expert radiologist using real-time linear-array scanner probes with a performance between 7.0 and 7.5 MHz. The study was restricted to bilateral Achilles tendon and quadriceps tendon because they provided the most efficient model for ultrasonographic analysis as a result of their superficial position and large size.7
We selected to use US to assess the Achilles tendon disease, because of its high accuracy in the diagnosis of various tendon disorders in addition to partial and full tendon ruptures.8 Focal or diffuse textural heterogeneity with the presence of hypoechoic areas, swelling, and calcifications, along with concomitant evaluation of the peritendon sheath and the surrounding peritendinous soft tissues for patchy thickenings, irregularities of tendon margins, and fluid collections, are the main findings of US. Quadriceps tendons’ rupture could also be diagnosed using ultrasound.9
Statistical Analyses
Data were analyzed using Statistical Program for Social Science version 18.0. Variables were first tested for normality using the Kolmogorov–Smirnov criterion. Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Pearson’s and Spearman’s rank correlation coefficient (r) tests were used for correlating data. The significance level was set at P < 0.05.
Results
The studied group of patients was 27 females and 23 males. Their mean age was 51.58 ± 12.5 years with a dialysis duration of 4.05 ± 2.11 years. The most common cause of renal failure in this group of patients was hypertension (26%) and then diabetes (22%), followed by chronic pyelonephritis (14%), chronic glomerulonephritis (Ch.GN) (10%), autosomal dominant polycystic kidney disease (ADPKD) (8%), renal obstruction (8%), unknown cause (8%), and analgesic abuse (4%), which was the least one.
Local examination of knees and ankles of the studied group showed that the most frequent sign was tenderness in ankles, 18 (36%) patients, followed by knees, 6 (12%) patients, and unilateral knee swelling, only 1 (2%) patient.
Ultrasonograhic examination of the Achilles tendon in the studied group showed that tenderness during probing was the most frequent finding, 22 (44%) patients, followed by calcific deposition, 12 (24%) patients; abnormal peritendon tissue for patchy thickenings, irregularities of tendon margins, and fluid collections, 10 (20%) patients; abnormal anteroposterior (A-P) middle one-third thickness of Achilles tendon >6 mm, 10 (20%) patients; abnormal A-P of distal one-third thickness of Achilles tendon >6 mm, 9 (18%) patients; and abnormal tendon structure (distorted tendon echostructure), 2 (4%) patients, which was the least one (Table 1). There were no cases of spontaneous tendon rupture or quadriceps tendon abnormality by ultrasound examination.
Table 1.
Ultrasound examination of Achilles tendons of the patients in the studied group.
| FINDING | NO=50 (%) | |
|---|---|---|
| Ultrasound examination | Tenderness during probing. | 22 (44) |
| Calcific deposit. | 12 (24) | |
| Abnormal peritendon tissue. | 10 (20) | |
| Bilateral A-P middle 1/3 thickness of Achilles tendon (>6 mm). | 10 (20) | |
| Bilateral A-P distal 1/3 thickness of Achilles tendon (>6 mm). | 9 (18) | |
| Abnormal tendon structure. | 2 (4) |
The correlation of different abnormal ultrasound examination findings of the Achilles tendon in the studied group with age, sex, CBC, serum creatinine, and BUN showed no statistically significant correlation (P > 0.05), but there was a statistically significant positive correlation between the presence of calcific deposit, and the duration of dialysis (P = 0.003) and serum level of calcium (P = 0.004), phosphorous (P = 0.001), and PTH (P = 0.01). Also, there was a statistically significant positive correlation between the increased A-P thickness of middle or distal one-third of the Achilles tendon, and the duration of dialysis (P = 0.001) and serum level of calcium (P = 0.02), phosphorous (P = 0.02), and PTH (P = 0.002).
Correlation between the PTH level and different quantitative and qualitative data shows that that there was a statistically significant positive correlation with the duration of dialysis (P = 0.001), serum phosphorus level (P < 0.001), and some of ultrasound findings such as calcific deposit (P = 0.01) and the increased middle (P = 0.046) and distal (P = 0.03) one-third thickness of the Achilles tendon, but there was no statistically significant correlation with age, sex, CBC, serum creatinine, BUN, serum calcium, calcium phosphorous products, and abnormal peritendon tissue (Table 2).
Table 2.
Correlation between PTH and quantitative and qualitative parameters.
| PARAMETERS | PTH | |
|---|---|---|
| R | P-VALUE (SIGNIFICANCE) | |
| PEARSON CORRELATION COEFFICIENT | ||
| Age (years) | −0.072 | 0.617 (NS) |
| Duration of dialysis (years) | 0.452 | 0.001 (HS) |
| Hb (g/dl) | −0.007 | 0.962 (NS) |
| WBC’s (/cmm) | 0.207 | 0.149 (NS) |
| PLT (/cmm) | −0.066 | 0.648 (NS) |
| Serum Creatinine (mg/dl) | −0.054 | 0.711 (NS) |
| BUN (mg/dl) | −0.031 | 0.830 (NS) |
| Ca (mg/dl) | −0.052 | 0.719 (NS) |
| PO4 (mg/dl) | 0.764 | <0.001 (HS) |
| Ca x PO4 product | −0.130 | 0.368 (NS) |
| Abnormal A-P middle 1/3 (mm) | 0.283 | 0.046 (S) |
| Abnormal A-P distal 1/3 (mm) | 0.307 | 0.03 (S) |
| SPEARMAN’S RANK CORRELATION COEFFICIENT | ||
| Sex | −0.081 | 0.576 (NS) |
| Abnormal peritendon tissue | −0.166 | 0.249 (NS) |
| Calcific deposit | 0.362 | 0.01 (S) |
| Abnormal tendon structure | 0.257 | 0.071 (NS) |
Abbreviations: Hb, Hemoglobin; WBCs, white blood cells; PLT, platelets; BUN, blood urea nitrogen; Ca, calcium; PO4, phosphorous; NS, non-significant; S, significant; HS, highly significant.
Discussion
Renal failure is a major public health problem. It is associated with the increased prevalence of physical inactivity, reduced exercise capacity, and different grades of disability.1 Implementation of physical activity both during and after the dialysis session is warranted and useful to enhance quality of life and physical performance, and to reduce the risk of mortality and hospitalization.2
Bone alterations and soft-tissue calcifications are often encountered in patients with end-stage renal disease and have been comprehensively investigated. Less common musculoskeletal manifestations, such as spontaneous tendon ruptures, have been sporadically reported. Their etiology and predisposing factors remain unknown.7
Most of the systemic diseases traditionally implicated as causes of muscle and tendon disruption do not include CKD. Only sporadic cases of spontaneous tendon ruptures have been reported in patients with CKD undergoing long-term hemodialysis.5 We underwent an ultrasonographic evaluation of Achilles tendon and quadriceps tendon in CKD patients undergoing regular hemodialysis and identified tendon abnormalities.
The current study showed from the demographic data among the studied patients that their mean age was 51.58 years with female predominance (54%) over male (46%). This is agreed by Jungers et al.10 who found that there was a marked preponderance of males and a dramatic increase of incidence with age in both genders. It also showed that the mean duration of dialysis was 4.05 years, and is in agreement with Brountzos et al.7 who also found that the mean duration of dialysis was 4.6 years in their studied patients.
Hypertension was the most common cause of chronic renal failure (CRF) in our patients (26%) followed by diabetes mellitus (DM) (22%). This was disagreed by Levey et al.11 who showed that DM was the most common cause of CRF (35%) followed by hypertension (30%).
According to clinical and ultrasonographic examinations of studied patients, the current study found that the most frequent sign was ankle joint tenderness and the most common finding during ultrasound examination was tenderness during probing in the Achilles tendon (44%), followed by calcific deposits (24%), abnormal peritendon tissue (20%), abnormal A-P middle one-third thickness of Achilles tendon (20%), abnormal A-P distal one-third thickness Achilles tendon (18%), and abnormal tendon structure (4%), which was the least finding. This was in disagreement with Brountzos et al.7 who found that the most common finding was abnormal Achilles tendon structure (44.1%), followed by abnormal peritendon tissue (35.6%), abnormal A-P middle one-third thickness of Achilles tendon (32.2%), abnormal A-P distal one-third thickness of Achilles tendon (30.5%), calcific deposits (23.7%), and tenderness in patients during probing (11.9%).
We found that there was statistically no significant correlation between abnormal peritendon tissue of Achilles tendon and age, duration of dialysis, laboratory results, or any other US findings. But there was a highly significant positive correlation between the presence of calcific deposit and duration of dialysis, Ca and PO4 level with statistically significant positive correlation to PTH level. This was explained by Angel12 who said that both high and low rates of bone turnover finally result in the formation of structurally inferior demineralized or woven bone and marked hypercalcemia, which lead to accelerated vascular and soft-tissue calcification. It is now thought that this calcification process is an active cell-mediated procedure resembling osteogenesis in bone, rather than a passive precipitation of calcium and phosphorus complexes, although passive precipitation may be to a certain extent implicated in the development of intratendinous calcified foci.
Also there was a highly significant positive correlation between abnormal A-P middle and distal one-third thicknesses of Achilles tendon, and the PTH level and duration of dialysis with significant positive correlation to high Ca and PO4 levels. Kerimoglu et al.13 had also found a significant correlation of Achilles tendon thickness and duration of hemodialysis. Brountzos et al.7 showed that the mean duration of hemodialysis was significantly greater when a tendon abnormality was present. Peritendinous alterations, distorted tendon echostructure, increased tendon thickness, and pain during palpation all occurred in patients with a mean duration on hemodialysis of more than six years. These findings were in complete agreement with previous reports, which based on clinical observations speculated that musculoskeletal manifestations were more frequently seen in patients who have undergone long-term hemodialysis.3 Also, some investigators suggested primary tendon disease,14,15 since a variety of tendon structural abnormalities were noted in patients without bone erosion in the tendon insertion site,4,5 or abnormality at the tendon attachment to the bone.16 As already reported, the presence of the uremic condition leads to the development of ROD, whose range fluctuates widely from high to low turnover states. Also, Weerakkody and Gaillard17 reported that hyperparathyroidism is one of Achilles tendon tear causes that presents by pain and swelling in the Achilles region, and the ultrasound shows enlargement of the tendon thickness with abnormally hypoechoic regions, which is mostly associated with tendinosis, or may show separation of the tear ends with a contour change of the tendon in case of full-thickness tear.
The uremic condition leads to the progressive retention of a large number of compounds called uremic retention solutes, toxins that normally are excreted by healthy kidneys. Although the exact role of these toxins such as indoxyl sulfate and osteoprotegerin has not been fully elucidated, previous studies have shown that, when accumulated in blood because of renal failure, they are capable of modifying bone metabolism and, thus probably, collagen metabolism.18 Such mechanisms could eventually explain the pathogenesis and etiology of our findings.19
No tendon rupture occurred in our patients during a mean duration of hemodialysis of approximately four years. In fact, all published case reports on spontaneous tendon rupture in hemodialysis patients suggested that the mean duration of hemodialysis exceeds 10 years in these patients.20 Since tendon ruptures rarely occur outside the setting of predisposing degenerative changes that weaken the strength of intratendinous structure, they believe that a significantly thickened and degenerated tendon over an underlying uremic condition should be diagnosed and viewed as an advanced case of tendinopathy.21
On studying the relation between PTH and quantitative and qualitative parameters, we found that there was no significant correlation between the age of the patient and the level of PTH. This result was opposed by Kiss et al.22, who found that there was an inverse correlation between age and serum PTH level. In this study, there was a positive correlation between the PTH level and the duration of dialysis. This is in agreement with Nasri and Kheiri23 study, which showed that there was also a positive correlation between the PTH level and the duration of dialysis treatment. Douthat et al.24 study results showed that PTH was positively correlated with hyperphosphatemia. Terai et al.25 study showed that there was an increase of vascular calcification in CKD patients with secondary hyperparathyroidism and hyperphosphatemia, which may be the same mechanism that leads to calcific deposition in tendons.
In conclusion, in CKD patients on regular hemodialysis, ankle affection was more common than knee affection. In ankle examination, ankle tenderness was the most frequent musculoskeletal sign, and the most common ultrasonographic finding was Achilles tendon tenderness during probing and then calcific deposits and abnormal peritendon tissue. The PTH level was positively correlated with duration of dialysis, and the presence of calcific deposit and the increased A-P middle and distal one-third thickness of Achilles tendons.
Future research should be done on a wide range of patients to support our findings and study the relationship between the level of iPTH and the presence of ROD. Also the effect of the long duration of dialysis on the patient’s tendons should be studied using US. Further large studies are needed to better understand the relation between the frequency and nature of the Achilles tendon and quadriceps tendon involvement in patients with renal failure undergoing regular hemodialysis, and the levels of PTH.
Figure 1.
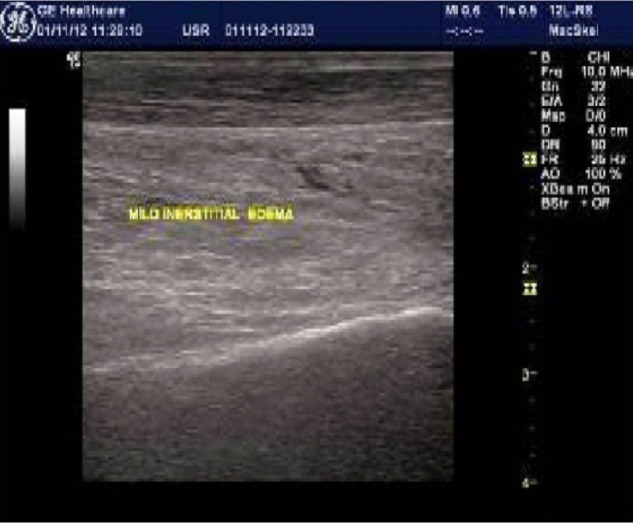
High-resolution ultrasound imaging identifies abnormal peritendon tissue in the form of interstitial edema.
Figure 2.
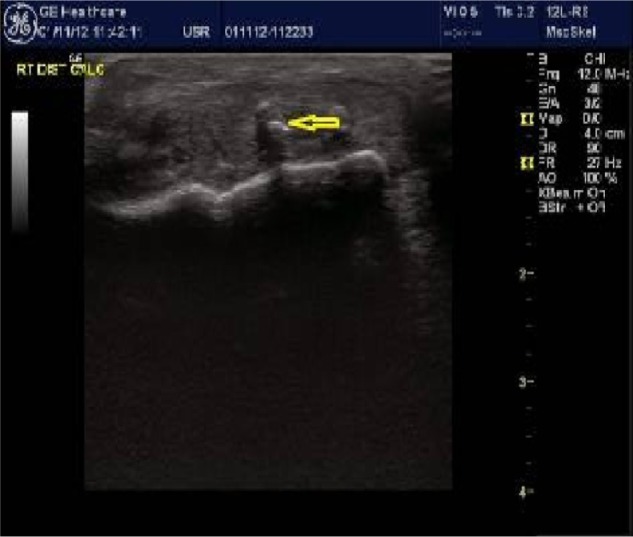
High-resolution ultrasound imaging identifies calcific deposits.
Figure 3.
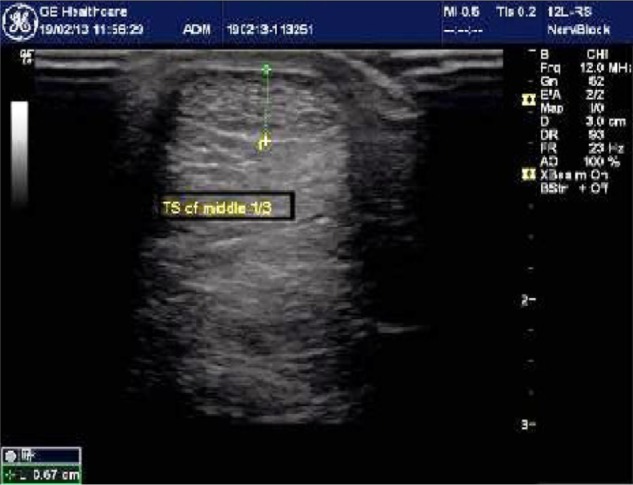
High-resolution ultrasound imaging of middle one-third of the Achilles tendon showing abnormal thickness of 6.7 mm.
Figure 4.
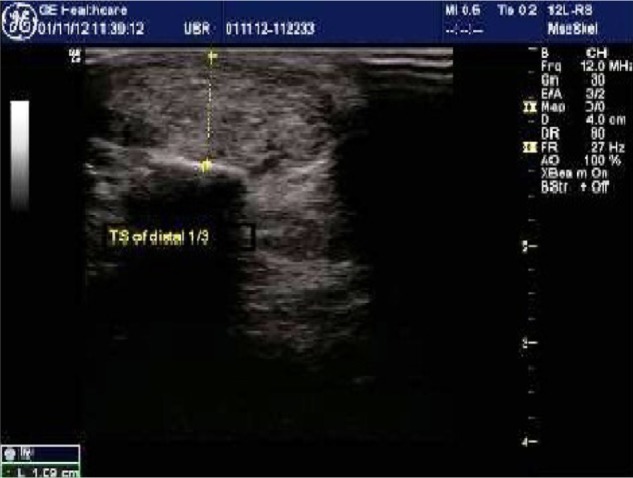
High-resolution ultrasound imaging of distal one-third of the Achilles tendon showing abnormal thickness of 10.9 mm.
Footnotes
Author Contributions
Conceived and designed the experiments: DAH, NOE. Analyzed the data: DAH, NOE, AHA, SAA, AMH, MHS. Wrote the first draft of the manuscript: NOE. Contributed to the writing of the manuscript: DAH, NOE, AMH, MHS. Agree with manuscript results and conclusions: DAH, NOE, AHA, SAA, AMH, MHS. Jointly developed the structure and arguments for the paper: NOE. Made critical revisions and approved final version: NOE. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Cupisti A, Capitanini A, Betti G, D’Alessandro C, Barsotli G. Assessment of habitual physical activity and energy expenditure in dialysis patients and relationship to nutritional parameters. Clin Nephrol. 2011;75:218–25. doi: 10.5414/cnp75218. [DOI] [PubMed] [Google Scholar]
- 2.Elder SE, Bommer J, Fissell RB. Hemodialysis facilities in which more patients exercise have lower risks of mortality and hospitalization: international results from the DOPPS. Am Soc Nephrol. 2005;16:94. [Google Scholar]
- 3.Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48–54. doi: 10.1097/00002281-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Basic-Jukic N, Juric I, Racki S, Kes P. Spontaneous tendon ruptures in patients with end-stage renal disease. Kidney Blood Press Res. 2009;32:32–6. doi: 10.1159/000201792. [DOI] [PubMed] [Google Scholar]
- 5.Chen CM, Chu P, Huang GS, Wang SJ, Wu SS. Spontaneous rupture of the patellar and contralateral quadriceps tendons associated with secondary hyperparathyroidism in a patient receiving long-term dialysis. J Formos Med Assoc. 2006;105:941–5. doi: 10.1016/S0929-6646(09)60180-7. [DOI] [PubMed] [Google Scholar]
- 6.Chew K, Stevens KJ, Wang TG, Fredericson M, Lew HL. Introduction to diagnostic musculoskeletal ultrasound: part 2: examination of the lower limb. Am J Phys Med Rehabil. 2008;87:238–48. doi: 10.1097/PHM.0b013e31816198c2. [DOI] [PubMed] [Google Scholar]
- 7.Brountzos E, Syrgiannis K, Panagiotou I, et al. Ultrasonographic alterations in Achilles Tendon in relation to parathormone in chronic hemodialysis patients. J Nephrol. 2009;22:476–83. [PubMed] [Google Scholar]
- 8.Archambault JM, Wiley JP, Bray RC, Verhoef M, Wiseman DA, Elliot PD. Can sonography predict the outcome of patients with achillodynia? J Clin Ultrasound. 1998;26:335–9. doi: 10.1002/(sici)1097-0096(199809)26:7<335::aid-jcu1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33:1983–90. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Jungers P, Chauveau P, Descamps-Latscha B, et al. Age and gender-related incidence of chronic renal failure in a French urban area: a prospective epidemiologic study. Nephrol Dial Transplant. 1996;11:1542–6. [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Angel LM. Secondary hyperparathyroidism: review of the disease and its treatment. J Clinthera. 2004;26:1976–93. doi: 10.1016/j.clinthera.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Kerimoglu U, Hayran M, Ergen BF. Sonographic evaluation of entheseal sites of the lower extremity in patients undergoing hemodialysis. J Med Ultrasound. 2007;35:417–23. doi: 10.1002/jcu.20411. [DOI] [PubMed] [Google Scholar]
- 14.Spencer JD. Spontaneous rupture of tendons in dialysis and renal transplant patients. Injury. 1988;19:86–8. doi: 10.1016/0020-1383(88)90079-4. [DOI] [PubMed] [Google Scholar]
- 15.Bhole R, Flynn JC, Marbury TC. Quadriceps tendon ruptures in uremia. Clin Orthop Relat Res. 1985;195:200–6. [PubMed] [Google Scholar]
- 16.Shiota E, Tsuchiya K, Yamaoka K, Kawano O. Spontaneous major tendon ruptures in patients receiving long-term hemodialysis. Clin Orthop Relat Res. 2002;394:236–42. doi: 10.1097/00003086-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Weerakkody Y, Gaillard F. Achilles tendon tear. Available from: http://radio-paedia.org/articles/achilles-tendon-tear.
- 18.Coen G, Ballanti P, Balducci A, et al. Serum osteoprotegerin and renal osteodystrophy. Nephrol Dial Transplant. 2002;17:233–8. doi: 10.1093/ndt/17.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Avbersek-Luznik I, Malesic I, Rus I, Marc J. Increased levels of osteoprotegerin in hemodialysis patients. Clin Chem Lab Med. 2002;40:1019–23. doi: 10.1515/CCLM.2002.177. [DOI] [PubMed] [Google Scholar]
- 20.Martinoli C, Derchi LE, Pastorino C, Bertolotto M, Silvestri E. Analysis of echotexture of tendons with US. Radiology. 1993;186:839–43. doi: 10.1148/radiology.186.3.8430196. [DOI] [PubMed] [Google Scholar]
- 21.Blankstein A, Cohen I, Diamant L. Achilles tendon pain and related pathologies: diagnosis by ultrasonography. Isr Med Assoc J. 2001;3:575–8. [PubMed] [Google Scholar]
- 22.Kiss I, Kiss Z, Ambrus C, et al. CKD-MBD Working Group of Hungarian Society of Nephrology Age-dependent parathormone levels and different CKD-MBD treatment practices of dialysis patients in Hungary – results from a nationwide clinical audit. BMC Nephrol. 2013;14:155. doi: 10.1186/1471-2369-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasri H, Kheiri S. Effects of diabetes mellitus, age, and duration of dialysis on parathormone in chronic hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19:608–13. [PubMed] [Google Scholar]
- 24.Douthat WG, Castellano M, Berenguer L, et al. High prevalence of secondary hyperparathyroidism in chronic kidney disease patients on dialysis in Argentina. Nefrologia. 2013;33:657–66. doi: 10.3265/Nefrologia.pre2013.May.12009. [DOI] [PubMed] [Google Scholar]
- 25.Terai K, Nara H, Takakura K, et al. Vascular calcification and secondary hyperparathyroidism of severe chronic kidney disease and its relation to serum phosphate and calcium levels. Br J Pharmacol. 2009;156:1267–78. doi: 10.1111/j.1476-5381.2008.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


