Figure 1.
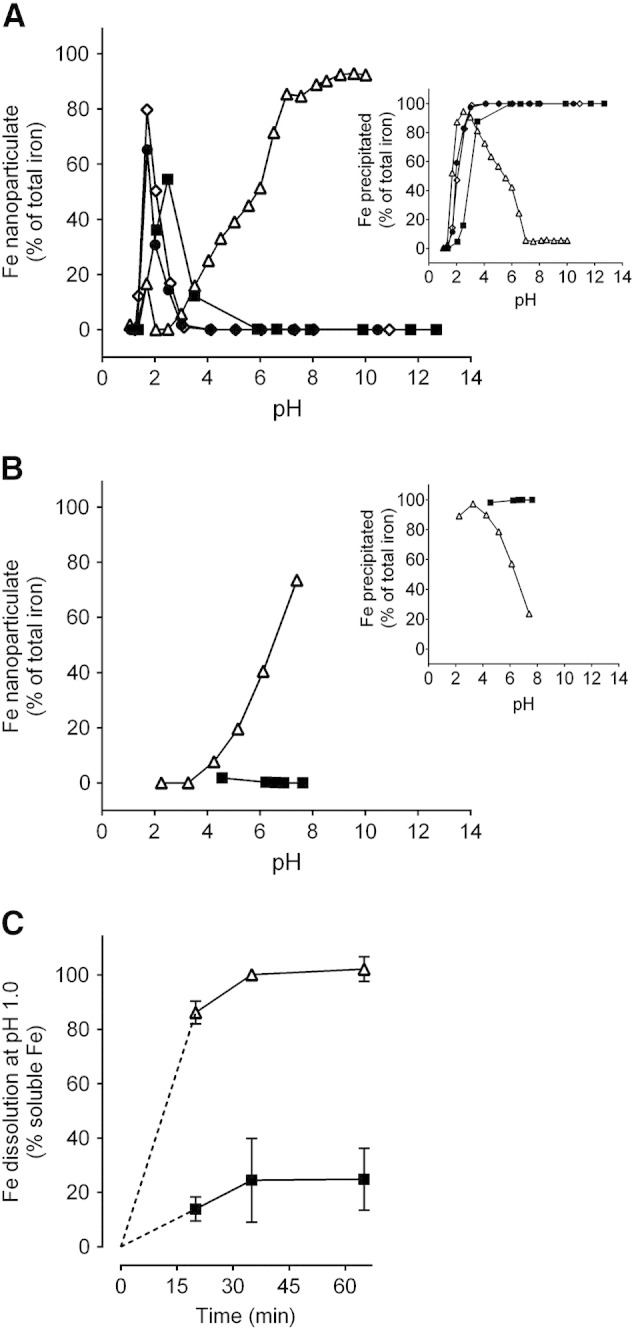
Formation of tartrate-modified ferrihydrite in adipate buffer as a function of pH. (A) Dispersed or colloidal (i.e. nanoparticulate) iron, determined following centrifugation and ultrafiltration to remove soluble iron (main panel), and precipitated (i.e. agglomerated) iron, determined following centrifugation (inset). Closed squares show synthetic ferrihydrite precipitated from an Fe(III) chloride solution; open triangles show modified ferrihydrite precipitated from an Fe(III) chloride solution in the presence of sodium tartrate and adipate buffer (Fe/tartrate/adipate = 1:0.5:0.5); closed circles and open diamonds show ferrihydrite precipitated from an Fe(III) chloride solution in the presence of adipate alone (Fe/adipate = 1:0.5 and 1:1 respectively). (B) Percentage of nanoparticulate iron (main panel) and precipitated iron (inset) for the synthetic (closed squares) and tartrate-modified (open triangles) ferrihydrite materials (molar ratios as above) re-suspended in the original volume of aqueous solution. (A and B) All values are expressed as a percentage of total iron in the initial solution as described in Methods. (C) Simulated gastric dissolution at pH 1.0 of dried synthetic ferrihydrite (closed squares) and tartrate-modified ferrihydrite that had been precipitated in the presence of tartrate and adipate (open triangles), at molar ratios as above, and then dried. Data were obtained following 5-min ultrafiltration (3000 Da MWCO); data are mean ± SD, n = 3.
