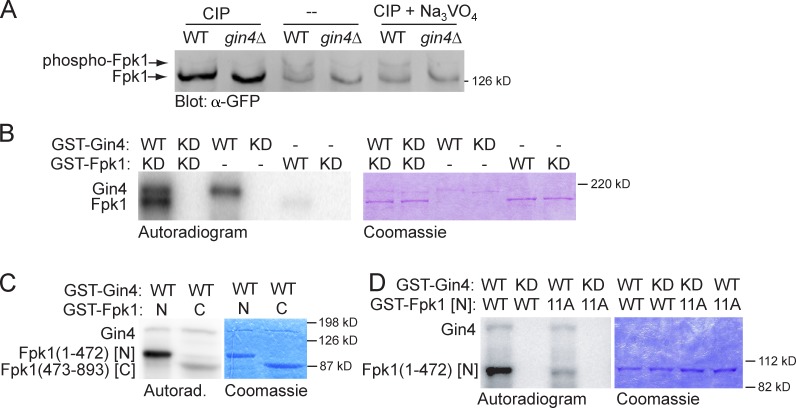
Figure 2.
Gin4 phosphorylates Fpk1 in vivo and in vitro. (A) Wild-type strain (YFR221, WT) or an isogenic gin4Δ (YFR224) expressing Fpk1-GFP were grown to mid-exponential phase and lysed. The resulting extracts were either not treated or treated with CIP or CIP + Na3VO4, resolved on a Phos-tag gel, and analyzed by immunoblotting with anti-GFP antibody. (B) GST-Gin4 (pAB1, WT) or catalytically inactive (“kinase-dead,” KD) mutant, GST-Gin4(K48A) (pAT103), were purified from E. coli, incubated with γ-[32P]ATP and either GST-Fpk1 (pFR143, WT) or catalytically inactive (KD) mutant GST-Fpk1(D621A) (pFR144), also purified from E. coli. Resulting products were resolved by SDS-PAGE and analyzed by autoradiography (left) and staining with Coomassie dye (right). (C) GST-Gin4 (pAB1, WT) was purified from E. coli, incubated with γ-[32P]ATP and either GST-Fpk1(1–472) (pBS1, N) or catalytically inactive GST-Fpk1(473–893; D621A) (pBS2, C), which were also purified from E. coli, and the products were analyzed as in B. (D) As in C, except that either GST-Gin4 (pAB1, WT) or catalytically inactive (KD) GST-Gin4(K48A) (pAT103) were incubated with either GST-Fpk1(1–472) (pBS1, WT) or GST-Fpk1(1–472; 11A) in which 11 Gin4 phosphorylation sites were mutated to Ala (pJW2).