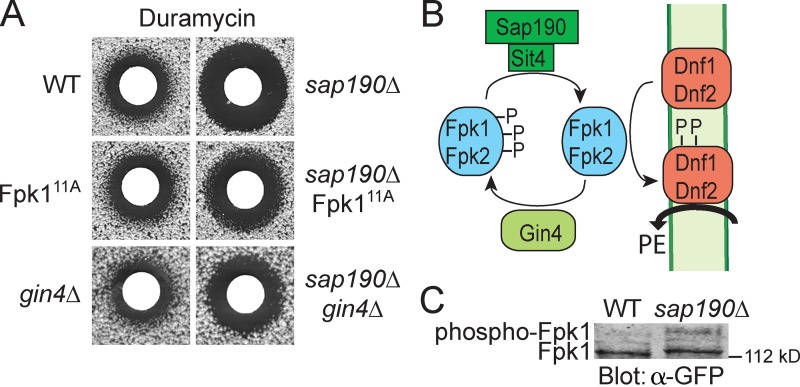
Figure 4.
Gin4 phosphorylation of Fpk1 results in down-regulation of flippase activity. (A) Wild-type (BY4741, WT), Fpk111A (YJW2), or gin4Δ (YAT100) cells, or isogenic derivatives of the same strains carrying a sap190Δ mutation (sap190Δ [YFR320], sap190Δ Fpk111A [YFR323], and sap190Δ gin4Δ [YFR326]), were plated as a lawn on YPD plates and then overlaid immediately at the center with a sterile filter paper disk onto which 10 µl of a stock solution of duramycin (8 mM) had been spotted. After incubation at 30°C for 3 d, plates were photographed. (B) Model for control of Fpk1 (and Fpk2)-dependent activation of flippases Dnf1 and Dnf2 by Gin4-mediated phosphorylation and Sit4-Sap190 dephosphorylation of Fpk1. Trapping of Fpk1 (and Fpk2) in their Gin4-phosphorylated state prevents optimal flippase function, increasing the level of PtdEth in the outer leaflet of the PM, thereby conferring greater sensitivity to duramycin. (C) Either wild-type cells (YFR221, WT) or an isogenic sap190Δ mutant (YFR328) expressing Fpk1-GFP were grown to mid-exponential phase and lysed. The resulting extracts were resolved on a Phos-tag gel and analyzed by immunoblotting with anti-GFP antibody.