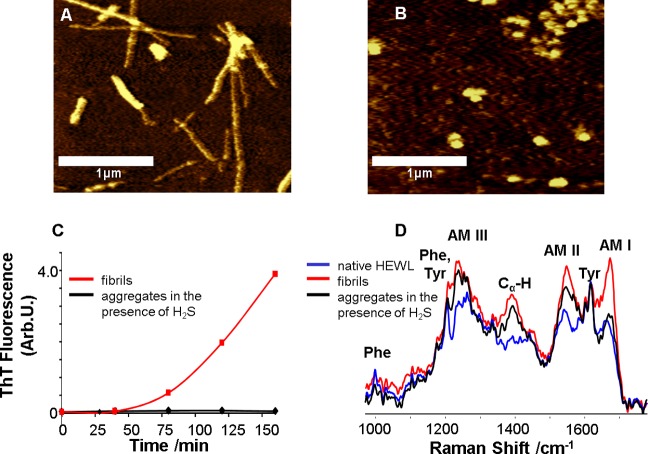
Figure 1.
Lysozyme forms β-sheet-rich fibrils under fibrillogenic control conditions and spherical aggregates of unordered protein under fibrillogenic conditions with H2S incubation. AFM images of (A) HEWL fibrils formed after incubation of the control solution for 90 min and (B) HEWL aggregates formed after incubation of the solution in the presence of H2S for 48 h; scale bars are 1 μm. (C) Aggregation kinetics (ThT fluorescence) of HEWL incubated under control conditions (red) and in the presence of H2S (black). (D) DUVRR spectra of native HEWL (blue), HEWL fibrils (red) and HEWL spherical aggregates (black) formed in the presence of H2S; all spectra were normalized using the aromatic amino acid Raman band (approximately 1600 cm–1) for comparison. The amide I vibrational mode (Am I) is dominated by C=O stretching, with minor contributions from C–N stretching and N–H bending.28 Amide II (Am II) and amide III (Am III) bands involve significant C–N stretching, N–H bending, and C–C stretching.29 The Cα–H bending vibrational mode involves Cα–H symmetric bending and C–Cα stretching.30