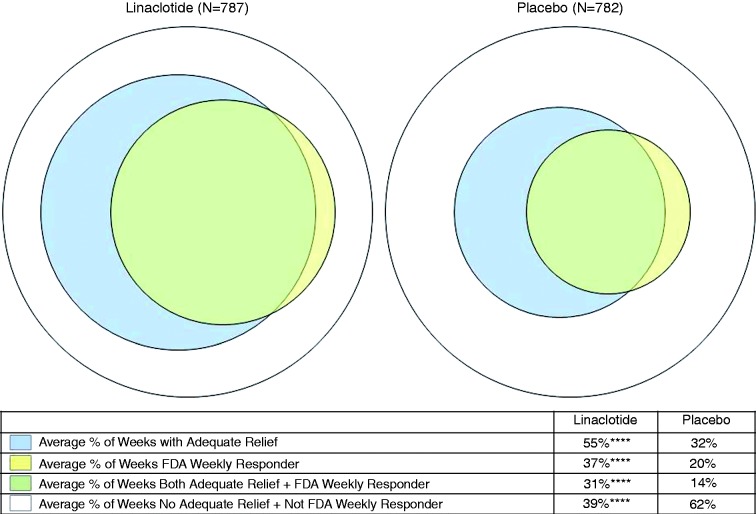
Figure 1.
Within-patient agreement between weekly adequate relief and weekly FDA responder criteria. Pooled phase 3 IBS-C ITT population, Weeks 1–12. ****p < 0.0001 for linaclotide vs placebo; p values were obtained from an ANOVA model with treatment group, geographic region, and trial as factors. Agreement (average % of weeks with AR and FDA weekly responder + no AR and not FDA weekly responder) = 70.2% for linaclotide and 76.4% for placebo; average % of weeks with AR and not FDA weekly responder = 24.0% for linaclotide and 18.2% for placebo; average % of weeks no AR and FDA weekly responder = 5.7% for linaclotide and 5.5% for placebo.
FDA: Food and Drug Administration; IBS-C: irritable bowel syndrome with constipation; ITT: intent to treat; ANOVA: analysis of variance; AR: adequate relief.