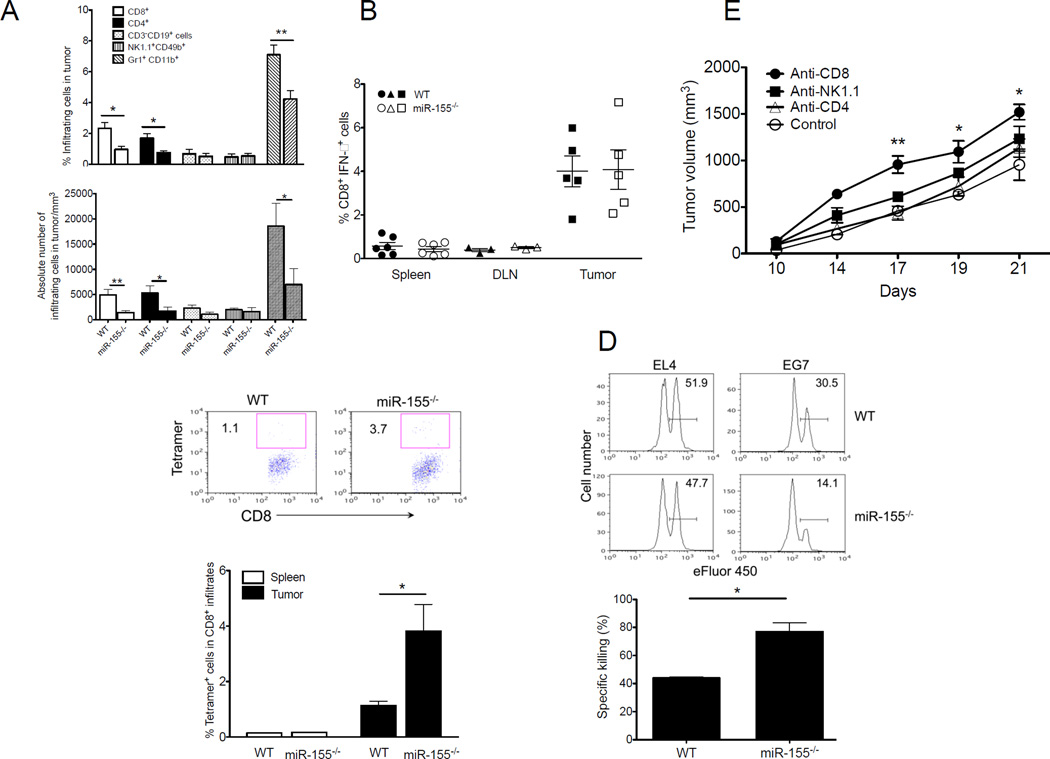
Figure 2. Host miR-155 deficiency enhances antigen-specific antitumor T cell immunity.
(A) Percentage and absolute number of CD4+CD3+, CD8+CD3+, Gr1+CD11b+, CD3−CD19+ and CD49b+NK1.1+ cells in tumor infiltrates of WT or miR-155−/− mice collected 21 days after inoculation with EG7 tumor cells (n=5). (B) CD8+IFN-γ+ T cell frequency in spleen, DLN and tumor from EG7-bearing WT or miR-155−/− mice 21 days after tumor inoculation (n=3–6). (C) Representative flow cytometric analysis of tumor antigen-specific CD8+ T cells from EG7-bearing WT or miR-155−/− mice. Frequency of tetramer+ cells specific for the OVA epitope SIINFEKL in CD8+ infiltrates from mice in B, was summarized. (D) Representative flow cytometric analyses of in vivo antigen-specific killing capacity of antitumor T cells from EL4- or EG7-bearing WT and miR-155−/− mice. Equal numbers of eFluor® 450high SIINFEKL peptide-pulsed and eFluor® 450low SIY–peptide-pulsed WT splenocytes were adoptively transferred into tumor-bearing mice. Numbers denote percentage of SIINFEKL peptide-pulsed target cell killing in DLN. Percent killing for EG7-bearing mice in DLN was calculated as described in Methods (n=3). (E) In miR-155−/− mice (n= 5), depletion of CD4+ T cells, CD8+ T cells, or NK cells was achieved by twice weekly i.p. injection of control Ig, anti-CD4, anti-CD8, or anti–NK1.1 depleting Abs, respectively, beginning 1 day prior to tumor challenge. Data are representative of 2 independent experiments. *, p< 0.05; **, p<0.01.