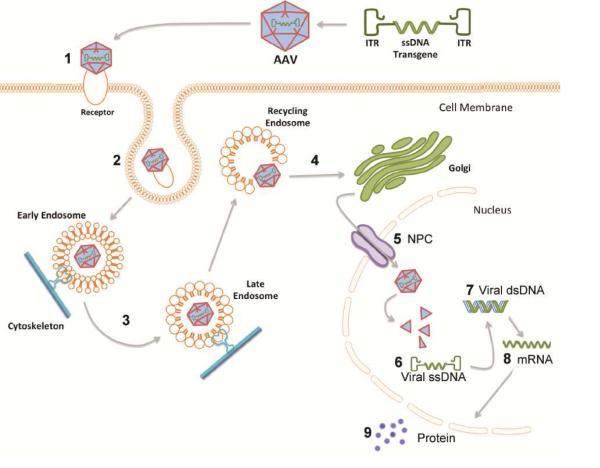
Figure 6. Steps of recombinant adeno-associated virus (rAAV) transduction and potential impact of the aged brain environment.
In viral transduction a single stranded DNA (ssDNA) expression cassette of interest is flanked by inverted terminal repeats (ITRs) to make up the recombinant genome that is packaged into rAAV capsid. 1) Injected rAAV capsids bind to designated cell surface receptor and co-receptors; rAAV2/5 binds with high affinity to 2,3-linked sialic acid glycan receptor (Walters et al, 2001) and platelet derived growth factor receptor (PDGFR) co-receptor (Di Pasquale et al, 2003). 2) Endocytosis of the viral particle occurs through clathrin coated pits or calveolar endocytosis; rAAV2/5 enters cell through clathrin-mediated endocytosis (Meier and Greber, 2004). 3) The rAAV capsid is trafficked through the cytoskeleton by dynamin in early, late, and recycling endosomes where acidification processes within the endosomes initiate capsid breakdown and genome release (Ding et al, 2005). 4) The viral particle is released from the endosome into the cytoplasm for golgi-mediated capsid processing (Bantel-Schall, Hub, and Kartenbeck, 2002). 5) The viral particle is internalized into the nucleus purportedly through the nuclear pore complex (NPC) (Hansen, Qing, and Srivastava, 2001). 6) The ssDNA genome is released from the viral capsid (Bartlett, Wilcher, and Samulski, 2000; Johnson and Samulski, 2009). 7) Viral ssDNA is converted to double stranded DNA (dsDNA) either by strand annealing or by second strand synthesis. 8) Viral dsDNA is converted into mRNA encoded by the transgene. 9) Transgene mRNA is translated into the protein encoded by the transgene. Numbers represent steps where viral transduction could be impeded by age-related cellular deficiencies (Bender et al, 2006; Blanpied, Scott, and Ehlers, 2003; D’Angelo et al, 2009; Gao et al, 2013; Kraytsberg et al, 2006; Park et al, 2001; Ryazanov and Nefsky, 2002; Sato et al, 2001; Sasaki et al, 2002; Smith, Sun, and Sokoloff, 1995). In the present set of experiments we demonstrate that robust deficits in exogenous transgene expression are associated with aging and that deficiencies leading to 4-fold less mRNA encoded by the transgene are primarily responsible for the diminished protein expression observed in the aged rats.