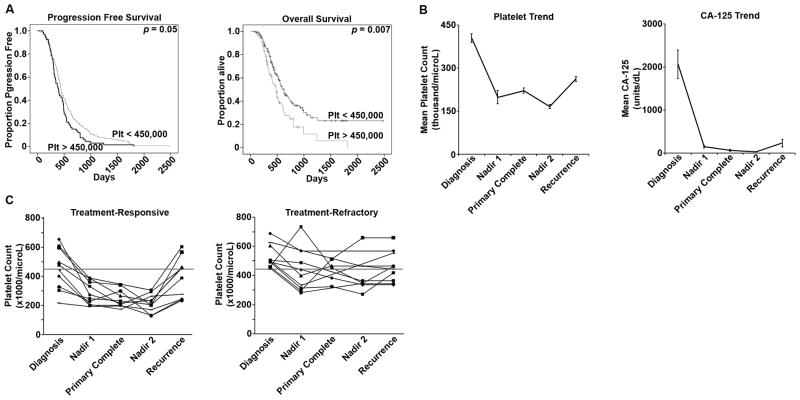
Figure 1.
A. Patients with recurrent ovarian cancer (n = 341) were identified and stratified according to their platelet counts at diagnosis into two groups: those with thrombocytosis (>450,000 cells/μL) and those with normal platelet counts (<450,000 cells/μL). The patients with thrombocytosis at the time of diagnosis had a significantly shorter median interval to progression (12.9 vs. 14.7 months, p = 0.05). The patients with thrombocytosis at the time of diagnosis had a significantly shorter median overall survival (16 vs. 20.8 months, p = 0.007).
B. Patients with recurrent ovarian cancer for whom longitudinal data was available through treatment and surveillance (n = 96). The mean platelet level at diagnosis was 403,000 cells/μL. During primary therapy, the mean platelet nadir was 198,000 cells/μL. At the conclusion of therapy, the average platelet level was 221,000 cells/μL, and this remained stable in the surveillance period, with the mean nadir 166,000 cells/μL during this time. At recurrence, mean platelet levels increased 27% to 262,000 cells/μL (p < 0.001). Of the patients with available longitudinal data, the mean level at diagnosis was 332 units/mL (normal <35 units/mL). Only 86% had a normal CA-125 level at the conclusion of primary therapy with a mean 63 units/mL, and the mean post-treatment nadir was 23 units/mL. At the clinical diagnosis of disease recurrence, CA-125 was elevated in 75% of patients, with a median 229 units/mL.
C. A subgroup of the patients with complete longitudinal data was identified who experienced progression of disease through first-line therapy (n = 10). These patients were matched to a cohort who experienced a durable response to therapy lasting more than 6 months. In the subgroup of patients with a durable response, only 50% had thrombocytosis (>450,000 cells/μL) at the time of diagnosis, and all patients in this subgroup achieved normal platelet counts during therapy. In the treatment-refractory cohort, all patients had thrombocytosis at the time of diagnosis, and platelet levels were more heterogeneous during primary therapy, with only 50% having normalized platelet counts by the completion of primary therapy.