Abstract
The early detection of lung cancer has the potential to greatly impact disease burden through the timely identification and treatment of affected individuals at a manageable stage of development. The insufficient specificity demonstrated by currently used screening and diagnostic techniques has led to intense investigation into biomarkers as diagnostic tools. Urine may represent a non-invasive alternative matrix for diagnostic biomarker development. We performed an analysis of 242 biomarkers in urines obtained from 83 NSCLC patients, 74 patients diagnosed with benign pulmonary conditions and 77 healthy donors. A large number of significant alterations were observed between the NSCLC and control groups. A multivariate analysis identified a three biomarker panel consisting of IGFBP-1, sIL-1Ra, CEACAM-1 which discriminated NSCLC from healthy controls with a SN/SP of 84/95 in an initial training set and 72/100 in an independent validation set. This panel performed well among multiple subtypes of NSCLC and early stage disease but demonstrated only limited efficacy for the discrimination of NSCLC from benign controls and limited specificity for several other cancers and tuberculosis patients. These findings demonstrate that urine biomarkers may provide screening and diagnostic properties which exceed those reported for serum biomarkers and approach a level necessary for further clinical development.
Keywords: NSCLC, screening, urine, biomarkers, Luminex
Introduction
Lung cancer is a devastating disease which accounts for more deaths in the US annually than prostate and breast cancer combined(1). Effective methods of early detection could dramatically reduce disease mortality and greatly benefit overall public health. Non-small cell lung carcinomas (NSCLC) represent the vast majority of lung cancers and while the overall five-year survival for patients with this diagnosis is a disappointing 15%, five-year survival for those patients diagnosed with stage IA NSCLC typically exceeds 60% (2). A number of techniques, including thoracic radiography, sputum cytology and computed tomography (CT), are currently being evaluated as diagnostic tools for lung cancer. While thoracic radiography and sputum cytology have failed to perform with adequate levels of sensitivity for early-stage disease in clinical trials [reviewed in Chanin et al. (3)], CT screening is now recommended for heavy smokers by the US Preventive Services Task Force (USPSTF)(4). The limitations of CT scanning are also well documented, including the high identification rate of benign pulmonary nodules (5, 6). Such findings greatly reduce the specificity of CT, exacerbating the already high cost of the technology and leading to unnecessary patient anxiety and surveillance. Thus remains the need to identify additional effective methodologies.
Investigations regarding the use of biomarker measurements as early detection tools for lung cancer have been conducted in serum, tissue and sputum, with serum being the least invasive and hence, most desirable testing matrix. Several serum biomarkers, including carcinoembryonic antigen (CEA), Cyfra 21-1, tissue polypeptide antigen (TPA), squamous cell carcinoma antigen (SCC), stem cell factor (SCF), granulocyte-macrophage colony stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF) have demonstrated associations with NSCLC, however each of these has failed to demonstrate the requisite sensitivity (SN) and specificity (SP) to warrant clinical development as diagnostic tools (7-11). A number of multianalyte panels comprised of both circulating proteins (12, 13) and tumor-associated autoantibodies (14, 15) have been evaluated with encouraging results. Recently, urine has been proposed as an alternative biofluid for analytical biomarker studies on the basis that the systemic information gained from such testing might be preserved while several of the limitations inherent to the use of blood could be eliminated. Urine is available in larger quantities than blood through less invasive means, allowing for repeated measurements aimed at patient surveillance or longitudinal studies. The urinary proteome is a direct product of renal filtration and consists of low molecular weight, soluble peptides which are highly amenable to proteomic analysis and may represent disease specific cleavage processes. Renal filtration also results in a less complex matrix than that of blood, containing fewer factors known to interfere with biomarker assays (16). Studies have shown that this proteome is stable for hours at room temperature, days at 4°C, and years at -20°C (17). What remains in the development of urine-based analytical platforms is evidence that systemic disease-specific biomarkers are released into this biological compartment in a manner which can be reliably measured and utilized for diagnostic means.
Effective biomarker based diagnostic tools have the potential to serve as alternatives or adjuncts to CT scanning for lung cancer. Investigators participating in the National Lung Screening Trial, a randomized multicenter trial involving more than 53,000 current and former smokers, recently released findings indicating a 20% reduction in lung cancer death in individuals screened by low-dose helical CT versus standard chest x-ray(18). These encouraging findings illustrate the promise offered by CT-based screening if certain limitations inherent to the technique can be overcome. The FDA has specified criteria for effective screening in reference to the use of CT in pulmonary cancer. Among these criteria, proposed by investigators at the Cleveland Clinic (19), is the requirement that any screening test directed at a disease with a prevalence of 5% or less must detect preclinical disease with a SN exceeding 95% when the SP is less than or equal to 95%, and vice versa. Current estimates place the prevalence of lung cancer in high-risk groups at 1-3% (6, 20), well below the 5% threshold, while the overall SN/SP of CT screening in this setting was recently reported at 90/92.6 (21). Thus, a biomarker-based test providing improved levels of SN/SP may successfully augment the performance of CT and provide a basis for targeted screening of high-risk groups, such as smokers. Alternatively, biomarker panels may be used in the triage of patients at increased suspicion of lung cancer to facilitate the timely referral of patients more like to be diagnosed with malignancy and reduce the level of unnecessary testing and surgery currently being performed.
Urines obtained from NSCLC patients, healthy controls, and individuals diagnosed with either benign lung abnormalities or pulmonary tuberculosis were evaluated using a large array of biomarker candidates. A bioinformatic analysis of the urine biomarker data identified a panel of three biomarkers capable of discriminating cases from controls with a high level of SN and SP.
Materials and Methods
Human Urines
Urines collected from 83 patients diagnosed with NSCLC, 74 patients diagnosed with a variety of benign lung conditions, 28 patients diagnosed with pulmonary tuberculosis (TB) and 77 healthy donors were obtained from Proteogenex Inc. (Culver City, CA) (Table 1, Supplementary Table S1). All subjects were over the age of 18 and provided written informed consent. Pregnant women and subjects with a history of blood borne illness were excluded. Urines were obtained prior to definitive treatment (surgery/chemotherapy/radiation) or on the day of surgery for NSCLC patients and benign controls. Diagnoses for NSCLC patients and benign controls were confirmed by clinical and pathological evaluation. Some benign controls were symptomatic of lung disease at the time of collection. The most commonly reported symptoms were weakness, cough, fatigue, fever, chest pain, dyspnea, and blood in sputum. Healthy donors were cancer free and free of pulmonary illness at the time of donation. All urines were spot collected at the time of medical visit or donation. Specific collection times were not reported or utilized in the current analysis. Urines were frozen at -70°C or -80°C within one hour of collection and remained frozen until testing. Each urine sample was annotated with information regarding age, gender, ethnicity, histology (NSCLC, benign, TB), stage (NSCLC), and smoking history. All patients and donors were Caucasian. The training and validation sets were received and tested separately.
Table 1. Clinical Characteristics of Study Population.
| Training | Validation | |
|---|---|---|
| Healthy | ||
| n | 49 | 28 |
| Age Range (Median) | 46-64 (57) | 41-65(56) |
| Male | 28 (57%) | 18 (64%) |
| Female | 21 (43%) | 10 (36%) |
| Smoking Status | ||
| Non-smoker | 16 (33%) | 22 (79%) |
| Smoker | 32 (65%) | 6 (21%) |
| Unknown | 1 | 0 |
| Mean Pack Years | 15 | 4 |
| NSCLC | ||
| n | 54 | 29 |
| Age Range (Median) | 38-77(65.5) | 48-79(64) |
| Male | 40 (74%) | 22 (76%) |
| Female | 14 (26%) | 7 (24%) |
| Smoking Status | ||
| Non-smoker | 10 (19%) | 4 (14%) |
| Smoker | 36 (67%) | 23 (79%) |
| Unknown | 8 | 2 |
| Mean Pack Years | 34 | 36 |
| Adenocarcinoma | 8 (15%) | 8 (28%) |
| Squamous cell carcinoma | 34 (63%) | 14 (48%) |
| BAC | 2 (3.5%) | 2 (7%) |
| Undifferentiated | 2 (3.5%) | 0 |
| NOS | 8 (15%) | 5 (17%) |
| Stage IA-IB | 17 (31%) | 10 (34%) |
| Stage IIA-IIB | 12 (22%) | 0 |
| Stage IIIA-IIIB | 14 (26%) | 9 (31%) |
| Stage IV | 4 (8%) | 6 (21%) |
| Stage unknown | 7 (13%) | 4 (14%) |
| Benign | ||
| n | 74 | |
| Age Range (Median) | 22-74 (52) | |
| Male | 48 (65%) | |
| Female | 26 (35%) | |
| Histology† | ||
| Benign neoplasm | 52 (20%) | |
| Cyst | 8 (11%) | |
| Other | 10 (14%) | |
| Unknown | 4 (5%) | |
| Symptomatic | 47 (64%) | |
| Asymptomatic | 27 (36%) | |
| Smoking Status | ||
| Non-smoker | 39 (53%) | |
| Smoker | 35 (47%) | |
| Mean Pack Years | 22 | |
| Pulmonary Tuberculosis | ||
| n | 28 | |
| Age Range (median) | 22-61 (36) | |
| Male | 19 (68%) | |
| Female | 9 (32%) | |
| Smoking Status | ||
| Smoker | 27 (96%) | |
| Non-Smoker | 1 (4%) | |
| Mean Pack Years | 11 | |
| Histology | ||
| Fibrous-cavernous | 12 (43%) | |
| Infiltrative | 5 (18%) | |
| Tuberculoma | 7 (25%) | |
| Focal | 1 (4%) | |
| Tuberculosis, NOS | 3 (11%) | |
| Symptomatic | 18 (64%) | |
| Asymptomatic | 10 (36%) |
BAC – bronchioalveolar carcinoma; NOS – not otherwise specified
complete breakdown of benign diagnoses provided in supplemental table S1
Urines obtained from patients diagnosed with breast (n=25) or prostate (n=25) cancer were utilized in the analysis of cancer specificity for selected biomarker panels. Urines were obtained from patients diagnosed with various stages of disease and were collected by the Health Sciences Tissue Bank at the University of Pittsburgh Medical Center (Pittsburgh, PA). All urines were collected and processed in the same manner as that described above.
Urine Biomarker Analysis
Urines were evaluated for levels of 242 cancer-related protein biomarkers (Table 2) using multiplexed bead-based immunoassays. The biomarker list was compiled based on a literature review of current proteins of interest within all fields related to lung cancer research. Biomarkers were selected from this list on the basis of suitable bead-based immunoassay availability. A total of 51 multiplexed panels were utilized in this study. Commercially available assays were obtained from Millipore (Billerica, MA), Novagen/EMD Chemicals (Gibbstown, NJ), and R&D Systems (Minneapolis, MN) and were performed according to manufacturer instructions. The remaining multiplexed panels were developed according to strict quality control standards by the UPCI Luminex Core Facility (22) and were performed as described previously (23), with the exception that all urine samples were run undiluted. All biomarker testing was performed on undiluted samples immediately upon thawing without concentration of proteins or other pretest manipulation. Urines included in the training set were tested for the complete set of 242 analytes. Benign controls, TB patients, prostate cancer patients, and breast cancer patients were tested for a subset of 35 analytes. The selection of analytes for inclusion within this subset began with those biomarkers identified as most useful in the training set. A number of additional analytes of interest were also evaluated based on their inclusion in multiplexed panels utilized above. For the analysis involving TB patients, the training and validation sets were combined for the NSCLC and Healthy groups in order to increase the power of the study. Urine creatinine (UCr) levels were determined for each sample using the Creatinine Parameter Assay Kit (R&D Systems, Minneapolis, MN) and were used to evaluate the need for normalization of urine biomarker levels to account for variation in fluid intake.
Table 2. Complete List of Multiplexed Analytes.
| Inflammation | 6Ckine1, Adiponectin1, BCA-11, BLC/CXCL131, CCL14α/HCC-11, CCL19/MIP3β1, CCL20/MIP3α1, CD137/4-1BB1, CD301, CD40L1, CTACK1, CXCL11/I-TAC1, CXCL6/GCP21, CXCL7/NAP21, CXCL9/MIG1, ENA-781, Eotaxin 1,31*, Flt-3L1, Fractalkine1, G-CSF1, GM-CSF1, gp1301, I-3091, IL-1β,6,8,11,16,20,21,23,28α,29,331*, IL-1RI,1RII,2Rα,4R,6R1*, INF-β1, INF-ω1, IP-101, LIF1, MCP-1,2,41*, M-CSF1, MIF1, MIP-1δ, 41*, MPO2, NGAL1, OC1, OPG1, OPN1, OSN1, Perforin1, RAGE1, RANKL1, RANTES1, SCF1, SDF-1α+β1, TARC1, TNF α,β1*, TNF RI, RII1*, TSLP1, XCL1/Lymphotactin1 |
| Growth/Angiogenesis | Amphiregulin2, Ang-24, Angiogenin2, Angiostatin4, ANGPTL 3,4,61*, BDNF1, Betacellulin2, CD-1054, CNTF1, EGF2, EGFR2, Endostatin4, Epiregulin2, ErbB24, FGF-19, 21, 231*, FGF-β2, HB-EGF2, HGF1, IGF-1R2, IGFBP- 1-71*, NGF1, PDGF-AA, BB, AA/BB1*, TGFα1, Thrombomodulin2, Thrombospondin4, VEGF2, VEGFR 1-31* |
| Tumor Markers | AFP1, CA-1254, CA15-34, CA19-94, CA72-44, CEA4, Cytokeratin194, EPCAM4, fPSA4, HE44, HER-22, Mammaglobin4, Mesothelin4, tPSA4, SCC4 |
| Endocrine | ACTH1, alpha MSH1, Cortisol1, ERα2, FSH1, GH1, LH1, Melatonin1, NT-Pro-BNP2, Orexin A1, Oxytocin1, Progesterone Receptor2, Prolactin1, PTH1, TSH1 |
| Metabolism | AGRP1, Amylin(total)1, Apo AI, AII, B, CII, CIII, E1*, C-Peptide1, FABP11, Ghrelin1, GIP1, GLP-11, Glucagon1, H-FABP2, Insulin1, Leptin1, MDA-LDL2, PP1, PYY1, TPO1 |
| Serological | α2-Macroglobulin1, β2-Microglobulin1, Complement H, C3, C41*, CRP1, Ferritin1, Fetuin-A2, HSA1, PAI-12, Prealbumin1, SAA1, SAP1, vWF2 |
| Proteases/PIs | α1-Antitrypsin1, CathepsinD1, Kallikrein104, MMP-1-3,7-9,12,133*, TIMP 1-43*, tPAI-1(total)1, uPA2 |
| Apoptosis | Bcl-24, Granzyme A,B1*, Hif-1α4, sFAS1, sFASL1, TRAIL1 |
| Adhesion | ALCAM4, CEACAM-1,64*, E-Cadherin2, E-Selectin1, Fibronectin1, ICAM-11, NCAM1, PIGF2, Tenascin C2, VCAM-11 |
| Other | β-Endorphin1, Calbindin2, Clusterin1, CystatinC1, DKK-11, EN-RAGE2, GSTα2, GSTπ2, HSP27(Total)2, HSP602, HSP702, HSP90α2, Involucrin1, Keratin-1,10,111, Keratin-61, KIM-11, LOX-12, Lp(a)2, LPS1, MICA4, Neurotensin1, NSE4, Oncostatin4, PBEF4, PEDF1, pHSP27(Ser78/Ser82)2, Renin1, Substance P1, TFF-31, TgII4, THP2 |
Immunoassay suppliers:
Millipore,
Novagen/EMD,
R&D Systems,
UPCI Luminex Core Facility;
family members measured separately
Statistical Analysis
Biomarker measurements among the NSCLC and control groups were evaluated by the Mann-Whitney non-parametric U test. An initial minimum level of significance of p≤0.05 was utilized. The false discovery rate (FDR) was controlled at 5% according to the method of Benjamini and Hochberg (24). Biomarker results were normalized according to UCr levels by dividing the fluorescence intensity level for each biomarker measurement by the UCr level expressed in mg/dL. Correlations in biomarker levels were examined using the Pearson test for correlation in Graphpad Prism (La Jolla, CA).
Multivariate Analysis
A Metropolis algorithm with Monte Carlo optimization (MMC) was used for the multivariate analysis of the biomarker results as described previously (25). Briefly, biomarker combinations of a predetermined size are randomly assembled. A scoring function (SF) is then calculated for each biomarker panel as a linear combination of biomarker concentrations multiplied by a coefficient for each biomarker assigned by Monte Carlo optimization. The resulting set of SFs for each biomarker combination are then evaluated for classification efficiency using 10% cross-validation. In order to avoid overfitting bias, our analysis was limited to panels consisting of 2, 3, or 4 biomarkers. Panels were evaluated based on SN at predetermined SP levels of 90% and 95%. A level of 95% SP was chosen for the discrimination of NSCLC patients from Healthy controls in order to identify models capable of minimizing false-positive results. All multivariate analysis was restricted to the training set until the highest performing panels were identified. Urines in the validation set were then tested for each of the biomarkers included in the top performing panels and the model was applied to the results. The predicted diagnosis for each sample, based on the derived scoring function, was compared to the actual diagnosis to evaluate the accuracy of the model. Receiver operating characteristic (ROC) curves were constructed from the algorithm results and the area under the ROC curve (AUC) was determined using GraphPad Prism (La Jolla, CA). Due to concern that discrepancies in smoking history between the case and control groups may contribute to biomarker panel performance, the MMC algorithm was applied to the biomarker results in a separate analysis which included pack years (PY) as an independent variable.
Results
Urine Biomarker Analysis
When the biomarker measurements were analyzed with the FDR controlled at 5%, 37 biomarkers were found to be significantly altered between the NSCLC patients and Healthy controls with a maximum p-value of 0.0037 (Table 3). Ferritin and CRP were the most significantly altered biomarkers in this analysis, demonstrating elevated levels in NSCLC patients, followed by thrombospondin which was significantly decreased in the NSCLC group. CEACAM-1 was also among the most significantly altered biomarkers, demonstrating elevated levels in NSCLC patients. Among all of the significantly altered biomarkers, roughly half were observed to be increased in the NSCLC group while half were decreased. When the NSCLC and Benign groups were compared, only 3 biomarkers were observed to be significantly altered (Table 3). CEACAM-1 was the most significantly altered biomarker in this analysis, followed by ferritin and CRP. For each of these biomarkers, levels were significantly higher in NSCLC cases in comparison to the Benign controls. The distributions of the most significantly altered biomarkers in the NSCLC, Benign and Healthy groups are presented in Figure 1A.
Table 3. Urine Biomarker Levels in NSCLC Patients and Controls.
| Median Urine Levels | NSCLC vs Healthy† | NSCLC vs Benign† | ||||
|---|---|---|---|---|---|---|
| Marker | Units | Healthy | Benign | NSCLC | ||
| Ferritin | pg/ml | 2257 | 5916 | 15444 | 7.8×10-10 | 1.9×10-5 |
| CRP | pg/mL | 143 | 171 | 1265 | 1.7×10-9 | 2.4×10-5 |
| Thrombospondin | pg/mL | 27771 | 5983 | 2347 | 7.0×10-8 | NS |
| CEACAM-1 | pg/ml | 3199 | 4504 | 8112 | 2.2×10-7 | 1.7×10-5 |
| FGF-21 | pg/mL | 8.61 | 3.69 | 3.43 | 2.8×10-6 | NS |
| sCD30 | pg/mL | 5.39 | 6.39 | 6.56 | 4.4×10-6 | NS |
| Lp(a) | pg/mL | 0.773 | 0.979 | 0.915 | 5.0×10-6 | NS |
| TSH | uIU/mL | 0.087 | 0.138 | 0.197 | 8.0×10-6 | NS |
| PDGF-AA | pg/mL | 442 | 309 | 149 | 1.1×10-5 | NS |
| HE4 | pg/mL | 59738 | 102729 | 210120 | 1.6×10-5 | NS |
| MIF | pg/mL | 632 | 282 | 171 | 1.9×10-5 | NS |
| FSH | mIU/mL | 4.55 | 10.5 | 12.9 | 1.9×10-5 | NS |
| PBEF | pg/mL | 66259 | 54502 | 31156 | 9.0×10-5 | NS |
| Involucrin | pg/mL | 2213 | 597 | 408 | 1.1×10-4 | NS |
| OPG | pg/mL | 54.5 | 90.6 | 83.9 | 1.4×10-4 | NS |
| Endostatin | pg/mL | 1060 | 544 | 395 | 2.3×10-4 | NS |
| sIL-1Ra | pg/mL | 130 | 58.6 | 20.1 | 2.5×10-4 | NS |
| TNFB | pg/mL | 0.713 | 0.674 | 0.640 | 2.6×10-4 | NS |
| LPS | pg/mL | 24.5 | 20.1 | 19.8 | 3.7×10-4 | NS |
| Complement C3 | ng/mL | 1.43 | 0.833 | 0.280 | 5.0×10-4 | NS |
| IGFBP-1 | pg/mL | 79.4 | 220 | 232 | 8.3×10-4 | NS |
| C-Peptide | pg/mL | 13935 | 11462 | 11373 | 1.1×10-3 | NS |
| IL-6 | pg/mL | 1.04 | 3.67 | 2.30 | 1.2×10-3 | NS |
| Fibronectin | pg/mL | 3581 | 2735 | 2047 | 1.3×10-3 | NS |
| Kallikrein10 | pg/mL | 3058 | 2182 | 1415 | 1.4×10-3 | NS |
| AFP | pg/mL | 31.3 | 28.4 | 23.2 | 1.5×10-3 | NS |
| sCD137/4-1BB | pg/mL | 9.23 | 16.1 | 14.9 | 1.7×10-3 | NS |
| G-CSF | pg/mL | 0.292 | 0.316 | 0.251 | 1.7×10-3 | NS |
| TIMP-1 | pg/mL | 7.60 | 24.6 | 21.3 | 1.8×10-3 | NS |
| MMP-13 | pg/mL | 9.30 | 10.2 | 10.8 | 1.9×10-3 | NS |
| NGAL | pg/mL | 2353 | 3105 | 3446 | 2.0×10-3 | NS |
| Clusterin | pg/mL | 87916 | 152174 | 154297 | 3.0×10-3 | NS |
| HSP70 | pg/mL | 130 | 78.0 | 65.2 | 3.0×10-3 | NS |
| PDGF-AB/BB | pg/mL | 11.0 | 11.3 | 11.7 | 3.2×10-3 | NS |
| MMP-12 | pg/mL | 1.81 | 1.81 | 1.94 | 3.2×10-3 | NS |
| LIF | pg/mL | 8.81 | 9.64 | 9.54 | 3.6×10-3 | NS |
| CA72-4 | U/mL | 0.244 | 0.244 | 0.263 | 3.7×10-3 | NS |
p-value calculated by Mann-Whitney U-test with false discovery rate controlled at 5%;
NS – not significant
Figure 1. Top performing urine biomarkers and multimarker panel.
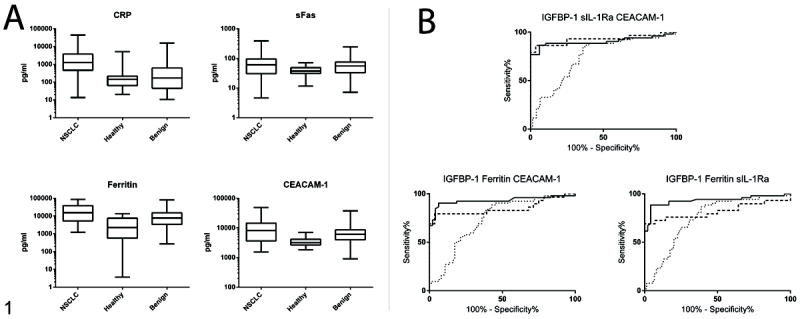
A. Box and whisker plots of top performing urine biomarkers. Levels of 235 urine biomarkers were measured in NSCLC patients (n=54), Healthy (n=49) and Benign (n=74) controls. Biomarkers demonstrating the strongest performance in the classification of cases vs. controls are shown. Boxes represent median with 25th and 75th percentiles. Whiskers represent maximum and minimum values. B. ROC analysis of selected multimarker panels. Performance of the top three 3-biomarker combinations in the classification of NSCLC patients from Healthy and Benign controls. Solid line: NSCLC vs Healthy training set; dashed line: NSCLC vs Healthy validation set; dotted line: NSCLC vs Benign.
The analysis of UCr-normalized data resulted in alterations among the majority of individual biomarker p-values, however the relative significance of each biomarker was largely unaffected. The vast majority of biomarkers demonstrated an increase in significance. CRP remained the most significantly altered biomarker in both the NSCLC vs. Healthy and NSCLC vs. Benign comparisons. Ferritin and CEACAM-1 also remained among the most highly altered biomarkers, however the significance of thrombospondin was markedly reduced. P-value alterations tended to be most severe among biomarkers of lesser significance (data not shown).
The TB patient urines were evaluated for a subset of 35 biomarkers and compared to the other experimental groups (Supplementary Table S2). Of the 35 evaluated biomarkers, 21 were observed to be significantly altered in the comparison of TB patients and Healthy controls. In the comparison of TB patients and NSCLC patients, 14 biomarkers were observed to be significantly altered. A separate set of 12 biomarkers was observed to be significantly altered in the comparison of the TB and Benign groups.
We noted a high degree of variability in the proportion of smokers and non-smokers among the NSCLC and control groups and among the training and validation sets (Table 1). To address this, urines from the training set were combined and redistributed on the basis of smoking status. Subjects of unknown smoking history were excluded. Each of the urine biomarkers listed in Table 3 was examined in these two groups as described in Materials and Methods. After controlling the FDR at 5%, none of the tested biomarkers were found to differ significantly between smokers and non-smokers. The effect of gender on urine biomarker levels was examined in the same manner. A total of 21 biomarkers were observed to differ significantly among males and females. Age was evaluated as a potential confounder using the Pearson test of correlation. Six biomarkers were observed to correlate with age (p<0.05). The complete list of biomarkers significantly associated or correlated with smoking status, gender and age is presented in Supplementary Table S3.
Biomarker Panel Analysis
When the MMC algorithm was applied to the training set data, the top performing two-biomarker combinations identified each included IGFBP-1 and either Kal10, ferritin, or sIL-1Ra. Each of these combinations outperformed the best individual markers in terms of SN, however ROC AUC values were comparable. The top performing three-biomarker panels each included combinations of IGFBP-1, ferritin, CEACAM-1 and/or sIL-1Ra and each provided similar performance in the training set. Each of these panels provided notably elevated levels of SN in comparison to the two-biomarker combinations and individual markers and AUC values which were similar or modestly increased in some cases. The top performing four biomarker combinations each included the combination of IGFBP-1, ferritin, CEACAM and either ALCAM or HE4. Both panels performed nearly identically in the Training Set and provided notable improvements in SN and AUC over the smaller panels and individual markers. Each of the top performing 2, 3 and 4 biomarker panels were further evaluated for performance in the discrimination of NSCLC and Benign controls. The complete results of this analysis are presented in Table 4. Overall, performance was marked diminished in this analysis in comparison to the discrimination of NSCLC and Healthy controls.
Table 4. Multimarker Panel Performance in the Classification of NSCLC Patients from Healthy and Benign Controls.
| NSCLC v Healthy (Training) | NSCLC v Benign | NSCLC v Healthy (Validation) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SN | SP | AUC (95% CI) | SN | SP | AUC (95% CI) | SN | SP | AUC (95% CI) | ||||
|
| ||||||||||||
| CRP | 57 | 95 | .931 (.885-.978) | 39 | 90 | .753 (.654-.852) | ||||||
| sFas | 57 | 95 | .917 (.861-.973) | 4 | 90 | .578 (.414-.642) | ||||||
| Ferritin | 62 | 95 | .856 (.785-.926) | 32 | 90 | .713 (.623-.803) | ||||||
| CEACAM-1 | 63 | 95 | .801 (.708-.893) | 22 | 90 | .685 (.590-.780) | ||||||
| IGFBP-1 | Kal 10 | 82 | 95 | .873 (.795-.952) | 82 | 58 | .737 (.645-.829) | |||||
| IGFBP-1 | Ferritin | 78 | 95 | .902 (.837-.968) | 78 | 58 | .727 (.638-.816) | |||||
| IGFBP-1 | sIL-1Ra | 75 | 95 | .872 (.797-.947) | 75 | 65 | .720 (.631-.810) | |||||
| IGFBP-1 | Ferritin | CEACAM-1 | 86 | 95 | .930 (.873-.988) | 86 | 57 | .736 (.649-.824) | 79 | 92 | .850 (.738-.962) | |
| IGFBP-1 | sIL-1Ra | CEACAM-1 | 84 | 95 | .905 (.837-.974) | 84 | 62 | .745 (.657-.832) | 72 | 100 | .926 (.848-1.00) | |
| IGFBP-1 | Ferritin | sIL-1Ra | 88 | 95 | .932 (.877-.987) | 88 | 57 | .751 (.665-.836) | 72 | 85 | .819 (.697-.940) | |
| IGFBP-1 | Ferritin | CEACAM-1 | ALCAM | 92 | 95 | .951 (.902-.999) | 92 | 50 | .743 (.657-.829) | |||
| IGFBP-1 | Ferritin | CEACAM-1 | HE4 | 92 | 95 | .954 (.906-1.00) | 92 | 49 | .756 (.672-.841) | |||
Each of the biomarkers panels identified using the MMC algorithm and listed in Table 4 were further evaluated for disease selectivity in patients diagnosed with a variety of benign pulmonary lesions, pulmonary TB, prostate cancer and breast cancer (Table 5). Several trends were observed in this analysis. The inclusion of Kal10 in two-biomarker combinations resulted in a high degree of disease selectivity, however this marker was not found to be useful in the classification of NSCLC vs. Healthy in larger panels. Ferritin was included in many of the high performing panels, however this marker was associated with poor disease selectivity. A general trend was noted wherein selectivity diminished as panel size increased, therefore although the identified four biomarker panels offered the highest cancer classification performance, disease selectivity was notably poor. Based on these findings, we concluded that the three biomarker panel comprised on IGFBP-1, sIL-1Ra, and CEACAM-1 provided the most efficacious combination of cancer classification and disease selectivity.
Table 5. Performance of Multimarker Panels in Non-NSCLC Conditions.
| Benign Lung | TB | Prostate Cancer | Breast Cancer | ||||
|---|---|---|---|---|---|---|---|
| Correctly Classified as Non-NSCLC (%)† | |||||||
| IGFBP-1 | Kal 10 | 58 | 50 | 88 | 64 | ||
| IGFBP-1 | Ferritin | 58 | 25 | 24 | 40 | ||
| IGFBP-1 | sIL-1Ra | 65 | 58 | 76 | 72 | ||
| IGFBP-1 | Ferritin | CEACAM-1 | 57 | 20 | 16 | 36 | |
| IGFBP-1 | sIL-1Ra | CEACAM-1 | 62 | 33 | 68 | 60 | |
| IGFBP-1 | Ferritin | sIL-1Ra | 57 | 25 | 28 | 44 | |
| IGFBP-1 | Ferritin | CEACAM-1 | ALCAM | 50 | 20 | 28 | 44 |
| IGFBP-1 | Ferritin | CEACAM-1 | HE4 | 49 | 20 | 16 | 16 |
Performance at 95% SP for NSCLC versus Healthy Controls;
TB – pulmonary tuberculosis
The panel of IGFBP-1, sIL-1Ra, and CEACAM-1 was further evaluated in the validation set where it provided a SN of 72% at a SP of 100% (Table 4). The two alternative 3 biomarker panels listed in Table 4 were also evaluated in the validation set based on their similar performance characteristics in the training set and among Benign controls. In the validation set the panel of IGFBP-1, sIL-1Ra and CEACAM-1 provided the highest AUC level. ROC curves demonstrating the overall performance of each panel in the training and validation sets as well as in the Benign controls are presented in Figure 1B. The selected panel was further evaluated for the ability to correctly classify specific subgroups of NSCLC patients and Benign controls (Supplementary Table S4). At a SP of 95%, the panel correctly classified 69% of adenocarcinomas, 88% of squamous cell carcinomas, and 75% of bronchioalveolar carcinomas (BAC) from the pool of healthy controls. This panel also correctly identified 23 out of 27 (85%) early stage (stage 1A/1B) cancers. In order to further evaluate the impact of smoking history on our analysis, the panel was applied separately to smokers and non-smokers within the NSCLC group and found to perform moderately better (83% vs 71% accuracy) among smokers. This panel also correctly identified 94% of male cases and 100% of female cases. The panel correctly classified 62% of the Benign controls and performed slightly better among symptomatic individuals within that group in comparison to asymptomatic individuals.
The MMC algorithm was also used to identify multimarker panels specifically able to discriminate NSCLC patients from Benign controls and TB patients from Healthy controls. The results of this analysis are presented in Supplementary Table S5. For the discrimination of NSCLC patients from benign controls, the two most useful individual biomarkers were CRP and MCP-2. The combination of these two biomarkers was identified by both algorithms as the top performing two-biomarker panel and provided significant improvement over either biomarker alone. The addition of a third biomarker (sIL-1Ra, TNF-α) to the CRP/MCP-2 combination resulted in further improvements in performance, however the performance characteristics of the two resulting 3-biomarker panels were nearly identical. Several 4-biomarker panels were evaluated which each included the combination of CRP, MCP-1 and TNF-α, however each resulted in only a modest improvement in SN. CRP was the highest performing individual biomarker for the discrimination of TB patients from Healthy controls. The combinations of CRP/MCP-2 and cortisol/MCP-2 performed equally well and offered significant improvement over CRP alone. The combination of cortisol, MCP-2 and IL-10 was identified as the best three-biomarker panel, however this panel did not demonstrate any improvement over the two-biomarker combinations.
When PY was included as an independent variable in the multivariate analysis of NSCLC cases and Healthy controls, it was not included among the 12 highest performing 2, 3, or 4-biomarker panels identified. In the comparison of NSCLC and Benign controls, PY was found to factor prominently among the two-biomarker combinations and was included in 4 out of the top 10 identified combinations (Supplementary Table S5). PY was not included among the 12 highest performing 3 or 4-biomarker combinations.
Discussion
Several urine biomarkers including CRP, ferritin, and sFas were found to perform particularly well on an individual basis in the discrimination of NSCLC from Healthy controls, but demonstrated limitations as multimarker panel components. This was not surprising, particularly with regard to CRP and ferritin, as these factors represent acute phase reactants likely to be associated with disease in a nonspecific manner. Two recent studies which utilized subject cohorts nested within the large prospective Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial concluded that elevated serum levels of CRP, alone or in combination with IL-6, were associated with a greater risk of lung cancer in prediagnostic samples (26, 27). In those studies, risk increased steadily with serum CRP levels and differed markedly among current and former smokers. Notably, the combination of CRP and IL-6, measured in urine in the current study, was not effective in the discrimination of NSCLC from Healthy controls (55%SN at 95% SP). Another recent study found increased ferritin levels in the sera of NSCLC patients along with elevated expression in tumor samples (28). In that study the authors concluded that the elevated serum ferritin levels were likely the result of inflammation and oxidative stress rather than body iron overload. This conclusion is in agreement with our finding that ferritin was considerably nonselective in the comparison of NSCLC to other disease states. Several in vitro studies in lung cancer cell lines have implicated sFas in tumor progression, immune evasion, response to chemotherapy and metastasis (29-32). Although sFas was included in several preliminary multimarker panels, these panels were not among those selected for highest performance.
The urine biomarkers CEACAM-1, sIL-1Ra and IGFBP-1 were selected for inclusion in our optimally performing panel. The adhesion molecular CEACAM-1 has been previously shown to mediate the formation of a pro-angiogenic tumor microenvironment which supports tumor vessel maturation in a transgenic mouse model (33). A separate study found CEACAM-1 expression in 81.3% of primary tumors from NSCLC patients with preserved expression in lymph node and hematogenous metastases which was negatively correlated with overall and progression-free survival (34). An association between NSCLC and serum CEACAM-1 levels has been reported recently (35). To the best of our knowledge, the current study is the first to report such an association regarding urine CEACAM-1 levels. The anti-inflammatory cytokine sIL-1Ra was found to be associated with increased lung cancer risk in a prospective analysis of patients enrolled in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) (36). This cytokine was also included in two multimarker panels capable of risk stratifying screen detected pulmonary nodules and discriminating NSCLC patients from a group of high risk individuals in separate studies (37, 38). A specific role or association for IGFBP-1 in NSCLC has yet to be described, although expression of this marker has been described in NSCLC cell lines on a limited basis (39, 40).
Our group previously reported on the use of serum biomarkers as screening tools for NSCLC in a similarly designed study (41), and a comparison of this and the current studies provides several noteworthy observations. MIF was an essential component of the top biomarker panels in the previous serum study, however although it was found to be significantly altered, it was not included in the top performing panels in the current study. In the previous study, MIF was significantly elevated in the sera of NSCLC patients with respect to controls while in the current study involving urine, the opposite trend was observed. The former observation is consistent with the emerging role of MIF in NSCLC as an autocrine/paracrine driver of tumor development. The present finding in urine may be indicative of increased retention of MIF by diseased tissues or of some mechanistic alteration in the renal filtration of MIF. A similar observation was made for sIL-1Ra, which was also found to demonstrate opposing trends in serum and urine. Several additional biomarkers including IGBPB-1, thrombospondin, HE4, and Complement C3 all displayed consistent trends in alterations between the two studies. Thus, the relationship between serum and urine biomarkers exhibits considerable complexity which appears to be incompletely explained by glomerular filtration.
The use of spot collected urines in the current study raises questions regarding the temporal reproducibility of biomarker measurements within individuals. The current study was not designed to evaluate this, however this question was addressed recently (42). In that study, variations in biomarker measurements were assessed in individuals on an intra- and inter-day basis. The level of variability was typically on the order of 25-100% and differed considerably among biomarkers. The degree to which this variability corrected upon creatinine normalization also varied considerably among evaluated biomarkers, and in several cases creatinine normalization resulted in an increase in variability. Although each of the biomarkers reported in that analysis were included in the current study, the biomarkers included in the IGFBP-1, sIL-1Ra, CEACAM-1 panel were not evaluated in the previous study. However, we would reasonably expect each of these biomarkers to display similar ranges of variability and further note that the magnitude of differences among cases and controls reported in the current analysis far exceeds the previously reported range of variability within individuals. Our observations regarding creatinine normalization appear to support this notion, as the most highly altered biomarkers remained highly significant after normalization, while biomarkers of lesser significance were affected more profoundly.
Our validated panel was only moderately selective in the discrimination of NSCLC from several other disease conditions including TB, breast cancer and prostate cancer, indicating a potential limitation to this type of screening approach. Our analysis also consistently demonstrated a higher degree of biomarker alteration and discriminatory performance when NSCLC cases were compared to Healthy versus Benign controls. We, therefore, conclude that non-specific pathological biomarker responses are contributing to our findings. Thus, further studies of this type will be necessary to identify additional biomarkers specific for NSCLC in order to expand the clinical utility of urine biomarker panels.
The IGFBP-1, sIL-1Ra, CEACAM-1 panel correctly identified 62% of the Benign controls and performed slightly better in symptomatic vs. asymptomatic individuals. The utility of a biomarker based test as a tool for the differential diagnosis in the primary care setting would be defined by its ability to stratify patients based on lung cancer risk in order to aid in referral decisions. The prevalence of lung cancer among symptomatic individuals referred for additional testing varies considerably based on patient demographics, the training of the attending physician, the availability of necessary medical equipment and facilities and other factors (43). If this prevalence is estimated to range from 5-30%, the panel would provide a positive predictive value (PPV) ranging from 10-49% and a negative predictive value ranging from 99-91%. The best alternative panels listed in Supplementary Table S5 provide a somewhat higher PPV range (27-88%) and lower NPV range (98-88%). Clearly, an improvement in PPV will be necessary in order to prevent a high level of unnecessary referrals and the attendant cost and patient anxiety. However, it is also clear based on the demonstrated NPVs that if implemented properly, biomarker tests of this type may provide means of ruling out unnecessary referrals among individuals who would have been referred for additional testing according to current guidelines.
The classification efficiency achieved by the panel of IGFBP-1, sIL-1Ra, CEACAM-1 in our study compares favorably with a number of recent findings in serum involving protein biomarkers, microRNAs, proteomic profiles and autoantibodies (13, 14, 38, 44, 45). The performance of this panel also exceeds that of our recently reported serum biomarker panel consisting of MIF, prolactin and thrombospondin which provided a SN/SP of 74/90 in a study of 164 NSCLC patients and healthy controls (41). The evaluation of candidate biomarker panels in early stage cancer patients is likely the most critical aspect in panel development, given the potential to produce a stage shift among cancer diagnoses and improvements in survival trends. In the current study, the panel of IGFBP-1, sIL-1Ra, CEACAM-1 performed well among the early stage NSCLC patients included. The limited number of early stage cancers included in our report does represent a limitation of the study, however it is important to note that early stage cancers represented a full one-third of all cases evaluated and the proportion of early stage disease included in the validation set was higher than that of the training set. We noted a difference in the distribution of smokers and non-smokers in our training and validation sets. However, based on our analysis of individual biomarkers and biomarker panels with respect to smoking history, the altered distribution does not appear to account for the difference in performance. Our analysis does indicate that smoking history factored significantly into efforts to identify biomarker panels useful in the comparison of NSCLC and Benign controls, and this supports our overall assessment that the urine biomarkers included in this study were not highly effective in the discrimination of NSCLC from Benign controls.
The performance of the IGFBP-1, sIL-1Ra, CEACAM-1 panel among NSCLC cases and healthy controls appears promising given the high levels of SN and SP achieved and the reproducibility of these characteristics in the validation set. The identified urine panel was accurate in each of the major NSCLC subtypes and among early stage disease, but was limited in the discrimination of NSCLC from Benign controls, tuberculosis patients, and other cancers. Further evaluation of this panel and other candidate urine biomarker panels should focus more heavily on early stage disease in order to confirm the performance reported here. The evaluation of urines obtained prior to diagnosis of NSCLC through prospective studies would further expand the translatability of these findings.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by NIH/NCI grants R01CA108990 (A. Lokshin) and R21CA143736 (A. Lokshin) and utilized the UPCI Biomarker Facility supported in part by award P30CA047904 (A. Lokshin).
Footnotes
COI: The authors declare that they have no conflicts of interest.
Contributor Information
Brian M. Nolen, Email: nolanb@upmc.edu.
Aleksey Lomakin, Email: aleksey@mit.edu.
Adele Marrangoni, Email: marram@upmc.edu.
Liudmila Velikokhatnaya, Email: velix@upmc.edu.
Denise Prosser, Email: prosserd@upmc.edu.
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: pp. 1975–2008. [Google Scholar]
- 2.Mulshine JL, Sullivan DC. Clinical practice. Lung cancer screening. N Engl J Med. 2005;352(26):2714–20. doi: 10.1056/NEJMcp042630. Epub 2005/07/01. [DOI] [PubMed] [Google Scholar]
- 3.Chanin TD, Merrick DT, Franklin WA, Hirsch FR. Recent developments in biomarkers for the early detection of lung cancer: perspectives based on publications 2003 to present. Curr Opin Pulm Med. 2004;10(4):242–7. doi: 10.1097/01.mcp.0000130321.11513.13. Epub 2004/06/29. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160(5):330–8. doi: 10.7326/M13-2771. Epub 2014/01/01. [DOI] [PubMed] [Google Scholar]
- 5.Welch HG, Woloshin S, Schwartz LM, Gordis L, Gotzsche PC, Harris R, et al. Overstating the evidence for lung cancer screening: the International Early Lung Cancer Action Program (I-ELCAP) study. Arch Intern Med. 2007;167(21):2289–95. doi: 10.1001/archinte.167.21.2289. Epub 2007/11/28. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178(9):956–61. doi: 10.1164/rccm.200802-336OC. Epub 2008/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buccheri G, Torchio P, Ferrigno D. Clinical equivalence of two cytokeratin markers in mon-small cell lung cancer: a study of tissue polypeptide antigen and cytokeratin 19 fragments. Chest. 2003;124(2):622–32. doi: 10.1378/chest.124.2.622. Epub 2003/08/09. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg AK, Lee MS. Biomarkers for lung cancer: clinical uses. Curr Opin Pulm Med. 2007;13(4):249–55. doi: 10.1097/MCP.0b013e32819f8f06. Epub 2007/05/31. [DOI] [PubMed] [Google Scholar]
- 9.Pastor A, Menendez R, Cremades MJ, Pastor V, Llopis R, Aznar J. Diagnostic value of SCC, CEA and CYFRA 21.1 in lung cancer: a Bayesian analysis. Eur Respir J. 1997;10(3):603–9. Epub 1997/03/01. [PubMed] [Google Scholar]
- 10.Rapellino M, Niklinski J, Pecchio F, Furman M, Baldi S, Chyczewski L, et al. CYFRA 21-1 as a tumour marker for bronchogenic carcinoma. Eur Respir J. 1995;8(3):407–10. doi: 10.1183/09031936.95.08030407. Epub 1995/03/01. [DOI] [PubMed] [Google Scholar]
- 11.Schneider J. Tumor markers in detection of lung cancer. Adv Clin Chem. 2006;42:1–41. doi: 10.1016/s0065-2423(06)42001-1. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 12.Molina R, Filella X, Auge JM, Fuentes R, Bover I, Rifa J, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24(4):209–18. doi: 10.1159/000074432. Epub 2003/12/05. [DOI] [PubMed] [Google Scholar]
- 13.Patz EF, Jr, Campa MJ, Gottlin EB, Kusmartseva I, Guan XR, Herndon JE., 2nd Panel of serum biomarkers for the diagnosis of lung cancer. J Clin Oncol. 2007;25(35):5578–83. doi: 10.1200/JCO.2007.13.5392. Epub 2007/12/11. [DOI] [PubMed] [Google Scholar]
- 14.Farlow EC, Patel K, Basu S, Lee BS, Kim AW, Coon JS, et al. Development of a Multiplexed Tumor-Associated Autoantibody-Based Blood Test for the Detection of Non-Small Cell Lung Cancer. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-09-3192. Epub 2010/06/24. [DOI] [PubMed] [Google Scholar]
- 15.Wu LL, Chang WJ, Zhao J, Yu YW, Tan X, Su T, et al. Development of Autoantibody Signatures as Novel Diagnostic Biomarkers of Non-Small Cell Lung Cancer. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0193. Epub 2010/05/27. [DOI] [PubMed] [Google Scholar]
- 16.Ye B, Skates S, Mok SC, Horick NK, Rosenberg HF, Vitonis A, et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin Cancer Res. 2006;12(2):432–41. doi: 10.1158/1078-0432.CCR-05-0461. Epub 2006/01/24. [DOI] [PubMed] [Google Scholar]
- 17.Schaub S, Wilkins J, Weiler T, Sangster K, Rush D, Nickerson P. Urine protein profiling with surface-enhanced laser-desorption/ionization time-of-flight mass spectrometry. Kidney Int. 2004;65(1):323–32. doi: 10.1111/j.1523-1755.2004.00352.x. Epub 2003/12/17. [DOI] [PubMed] [Google Scholar]
- 18.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. Epub 2011/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obuchowski NA, Graham RJ, Baker ME, Powell KA. Ten criteria for effective screening: their application to multislice CT screening for pulmonary and colorectal cancers. AJR Am J Roentgenol. 2001;176(6):1357–62. doi: 10.2214/ajr.176.6.1761357. Epub 2001/05/25. [DOI] [PubMed] [Google Scholar]
- 20.Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773–81. doi: 10.1148/radiol.2223010490. Epub 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 21.Toyoda Y, Nakayama T, Kusunoki Y, Iso H, Suzuki T. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br J Cancer. 2008;98(10):1602–7. doi: 10.1038/sj.bjc.6604351. Epub 2008/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UPCI Luminex Core Facility. Available from: http://www.upci.upmc.edu/luminex/index.cfm.
- 23.Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(4):981–7. doi: 10.1158/1055-9965.EPI-04-0404. Epub 2005/04/13. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 25.Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28(13):2159–66. doi: 10.1200/JCO.2008.19.2484. Epub 2010/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28(16):2719–26. doi: 10.1200/JCO.2009.27.0454. Epub 2010/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103(14):1112–22. doi: 10.1093/jnci/djr216. Epub 2011/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kukulj S, Jaganjac M, Boranic M, Krizanac S, Santic Z, Poljak-Blazi M. Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer. Med Oncol. 2010;27(2):268–77. doi: 10.1007/s12032-009-9203-2. Epub 2009/03/25. [DOI] [PubMed] [Google Scholar]
- 29.Fan C, Lin X, Wang E. Clinicopathological significance of cathepsin D expression in non-small cell lung cancer is conditional on apoptosis-associated protein phenotype: an immunohistochemistry study. Tumour Biol. 2012 doi: 10.1007/s13277-012-0338-y. Epub 2012/02/04. [DOI] [PubMed] [Google Scholar]
- 30.Mitani K, Nishioka Y, Yamabe K, Ogawa H, Miki T, Yanagawa H, et al. Soluble Fas in malignant pleural effusion and its expression in lung cancer cells. Cancer Sci. 2003;94(3):302–7. doi: 10.1111/j.1349-7006.2003.tb01437.x. Epub 2003/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulukaya E, Acilan C, Yilmaz M, Yilmaztepe-Oral A, Ari F, Zik B, et al. sFas levels increase in response to cisplatin-based chemotherapy in lung cancer patients. Cell Biochem Funct. 2010;28(7):565–70. doi: 10.1002/cbf.1689. Epub 2010/10/14. [DOI] [PubMed] [Google Scholar]
- 32.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188(12):5954–61. doi: 10.4049/jimmunol.1103466. Epub 2012/05/11. [DOI] [PubMed] [Google Scholar]
- 33.Gerstel D, Wegwitz F, Jannasch K, Ludewig P, Scheike K, Alves F, et al. CEACAM1 creates a pro-angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene. 2011;30(41):4275–88. doi: 10.1038/onc.2011.146. Epub 2011/05/03. [DOI] [PubMed] [Google Scholar]
- 34.Thom I, Schult-Kronefeld O, Burkholder I, Schuch G, Andritzky B, Kastendieck H, et al. Expression of CEACAM-1 in pulmonary adenocarcinomas and their metastases. Anticancer Res. 2009;29(1):249–54. Epub 2009/04/01. [PubMed] [Google Scholar]
- 35.Zhou MQ, Du Y, Liu YW, Wang YZ, He YQ, Yang CX, et al. Clinical and experimental studies regarding the expression and diagnostic value of carcinoembryonic antigen-related cell adhesion molecule 1 in non-small-cell lung cancer. BMC cancer. 2013;13:359. doi: 10.1186/1471-2407-13-359. Epub 2013/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105(24):1871–80. doi: 10.1093/jnci/djt309. Epub 2013/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly S, Rinewalt D, Fhied C, Basu S, Mahon B, Liptay MJ, et al. Development and validation of a plasma biomarker panel for discerning clinical significance of indeterminate pulmonary nodules. J Thorac Oncol. 2013;8(1):31–6. doi: 10.1097/JTO.0b013e31827627f8. Epub 2012/12/04. [DOI] [PubMed] [Google Scholar]
- 38.Farlow EC, Vercillo MS, Coon JS, Basu S, Kim AW, Faber LP, et al. A multi-analyte serum test for the detection of non-small cell lung cancer. Br J Cancer. 2010;103(8):1221–8. doi: 10.1038/sj.bjc.6605865. Epub 2010/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaques G, Kiefer P, Schoneberger HJ, Wegmann B, Kaiser U, Brandscheid D, et al. Differential expression of insulin-like growth factor binding proteins in human non-small cell lung cancer cell lines. European journal of cancer (Oxford, England : 1990) 1992;28A(11):1899–904. doi: 10.1016/0959-8049(92)90032-w. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 40.Reeve JG, Brinkman A, Hughes S, Mitchell J, Schwander J, Bleehen NM. Expression of insulinlike growth factor (IGF) and IGF-binding protein genes in human lung tumor cell lines. J Natl Cancer Inst. 1992;84(8):628–34. doi: 10.1093/jnci/84.8.628. Epub 1992/04/15. [DOI] [PubMed] [Google Scholar]
- 41.Nolen BM, Langmead CJ, Choi S, Lomakin A, Marrangoni A, Bigbee WL, et al. Serum biomarker profiles as diagnostic tools in lung cancer. Cancer Biomarkers. 2011 doi: 10.3233/CBM-2012-0229. Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolen BM, Orlichenko LS, Marrangoni A, Velikokhatnaya L, Prosser D, Grizzle WE, et al. An extensive targeted proteomic analysis of disease-related protein biomarkers in urine from healthy donors. PloS one. 2013;8(5):e63368. doi: 10.1371/journal.pone.0063368. Epub 2013/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton W, Sharp D. Diagnosis of lung cancer in primary care: a structured review. Family practice. 2004;21(6):605–11. doi: 10.1093/fampra/cmh605. Epub 2004/11/03. [DOI] [PubMed] [Google Scholar]
- 44.Foss KM, Sima C, Ugolini D, Neri M, Allen KE, Weiss GJ. miR-1254 and miR-574-5p: serum-based microRNA biomarkers for early-stage non-small cell lung cancer. J Thorac Oncol. 2011;6(3):482–8. doi: 10.1097/JTO.0b013e318208c785. Epub 2011/01/25. [DOI] [PubMed] [Google Scholar]
- 45.Wu L, Chang W, Zhao J, Yu Y, Tan X, Su T, et al. Development of autoantibody signatures as novel diagnostic biomarkers of non-small cell lung cancer. Clin Cancer Res. 2010;16(14):3760–8. doi: 10.1158/1078-0432.CCR-10-0193. Epub 2010/05/27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


