Figure 2.
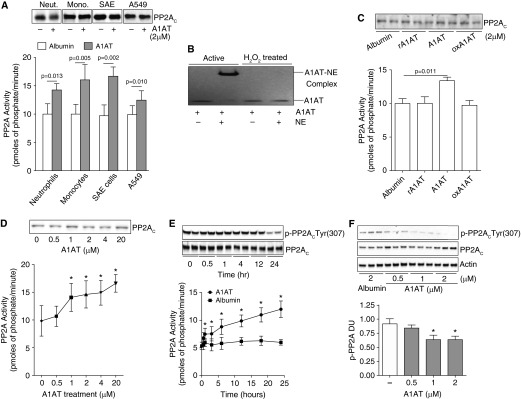
α1-Antitrypsin (A1AT) stimulation promotes cellular protein phosphatase 2A (PP2A) activity. (A) Treatment of human peripheral blood neutrophils and monocytes, human small airway epithelial (SAE) cells, and airway epithelial cell line A549 cells with 2 μM A1AT for 24 hours enhances PP2A activity. (B) Oxidized A1AT cannot form complexes with neutrophil elastase (NE) demonstrated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and could not (C) induce a PP2A cellular response in A549 cells. Recombinant/inactive A1AT (rA1AT) also had no effect on PP2A activity. (D) An A1AT dose response stimulation of A549 cells demonstrated that an A1AT concentration of greater than 1 μM significantly increases PP2A activity. (E) A1AT-induced PP2A activity was observed from 1 hour after stimuli with dephosphorylation of PP2AC greatest 24 hours after stimuli. (F) A1AT dephosphorylated PP2AC at Tyr site 307 in A549 cells. Densitometry analysis was performed for p-PP2AC(Tyr307) phosphorylation on immunoblots from 3 separate days and was represented as densitometry units (DU). Graphs represent mean ± SEM of three measurements from each subject (n = 6 per group). P values shown, comparing both treatments connected by a line. *P < 0.05 compared with baseline levels of activity. Representative PP2AC immunoblots are shown demonstrating equal cellular protein content.
