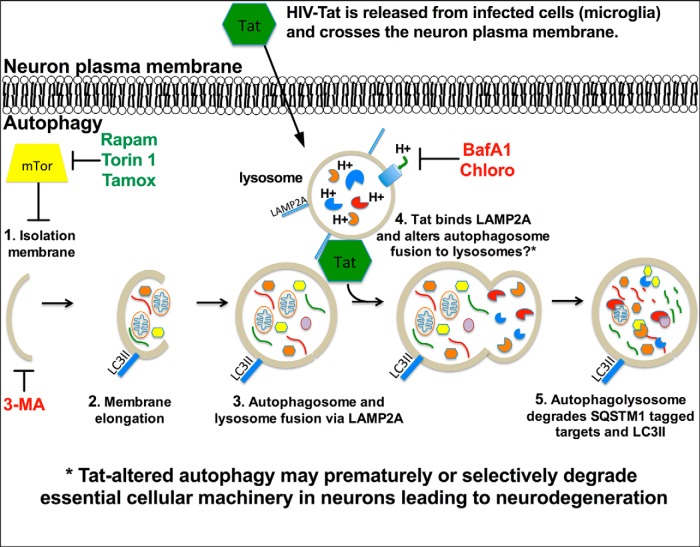
Figure 13.
Diagrammatic representation of the potential mechanisms through which Tat might interfere with neuronal autophagy. Early during HIV infection the virus crosses the blood–brain barrier in monocytes. These monocytes disseminate progeny virus, cytokines, and toxic viral proteins. Viruses infect other permissive CNS cells, such as microglia and possible astrocytes, and a cascade of viral replication and neuroinflammation ensues. HIV-1 Tat is secreted by infected cells and gains access to neurons; a phenomenon that may lead to bystander neuron malfunction or death. Autophagy is an essential cellular process that allows neurons to maintain dynamic synapses and respond to stress and learning cues. Autophagy begins with (1) initiation of the phagophore (induced by Rapam, Torin 1, and Tamox; inhibited by 3MA), which is inhibited by mTor, and (2) then elongation and engulfment of targeted cytosolic components. (3) The autophagosome fuses with the lysosome (inhibited by BafA1 and Chloro), via interaction with LAMP2A, to form the autophagolysosome and promote degradation of the autophagic cargo. (4) Our data suggest Tat associates with LAMP2A and thereby alters autophagolysosome formation or CMA progression. This scenario may explain increased LC3II and SQSTM1 degradation while increasing cytoplasmic autophagosome numbers. Alternatively, Tat may inhibit LC3II formation by an unknown mechanism, while concomitantly increasing degradation of SQSTM1-conjugated proteins through CMA. (5) The final stage of autophagy includes degradation of the autophagolysosome, internal cargo, and associated proteins such as LC3II and SQSTM1-conjugated proteins. Tat may influence HAND by increasing autophagy progression and causing premature degradation of proteins essential for neural activity.