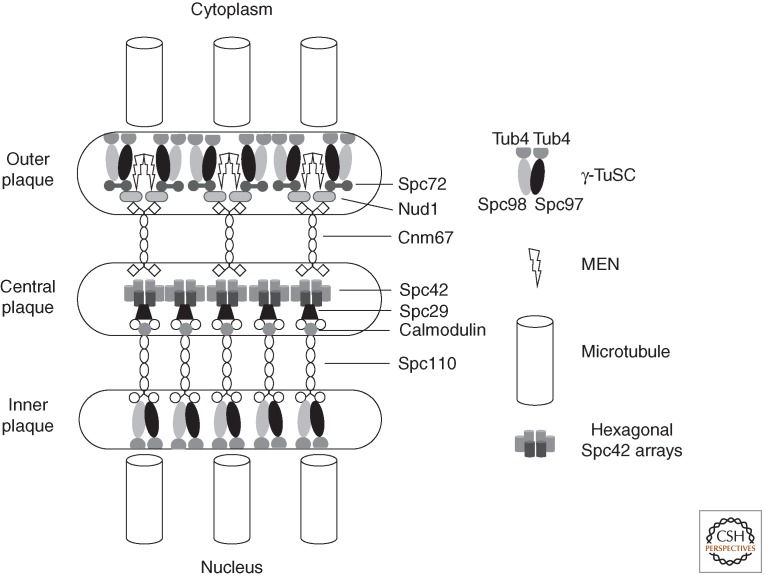
Figure 2.
A highly schematic representation of molecular architecture of the budding yeast spindle pole body (SPB). A hexagonal crystalline array of Spc42 units associate with Spc29/Spc110 complexes on the nuclear side and cnm67 dimers on the cytoplasmic side of the SPB. These spacer proteins separate the central Spc42 plaque from the γ-TuSC microtubule-nucleating centers at the inner and outer plaques. At the inner plaque the interaction between the spacer Spc110 is direct with one Spc110 dimer associating with a single γ-TuSC (Erlemann et al. 2012). It is estimated that a functional microtubule nucleation unit comprises seven γ-TuSCs, two additional Spc98, and three extra γ-tubulins (Erlemann et al. 2012). This estimate agrees well with the reconstitution of 13-fold symmetric γ-tubulin microtubule-nucleating units in vitro (Kollman et al. 2008, 2010). At the cytoplasmic outer plaque, the association between the spacer and the γ-TuSC is mediated through the association of Nud1 with Spc72. Despite the fact that Spc72 interacts with both Spc97 and Spc98 in two hybrid assays (Knop and Schiebel 1998), in vivo measurements suggest that one Spc72 dimer interacts with a single γ-TuSC (Erlemann et al. 2012). Nud1 also acts as a scaffolding molecule for the mitotic exit network (MEN) that couples the SPB position with cell-cycle control. The stoichiometries of other associations remain to be established. The representation of Spc29 in between Spc110 and Spc42 is highly schematic, as the exact nature of its function as part of the Spc110 complex remains to be established.