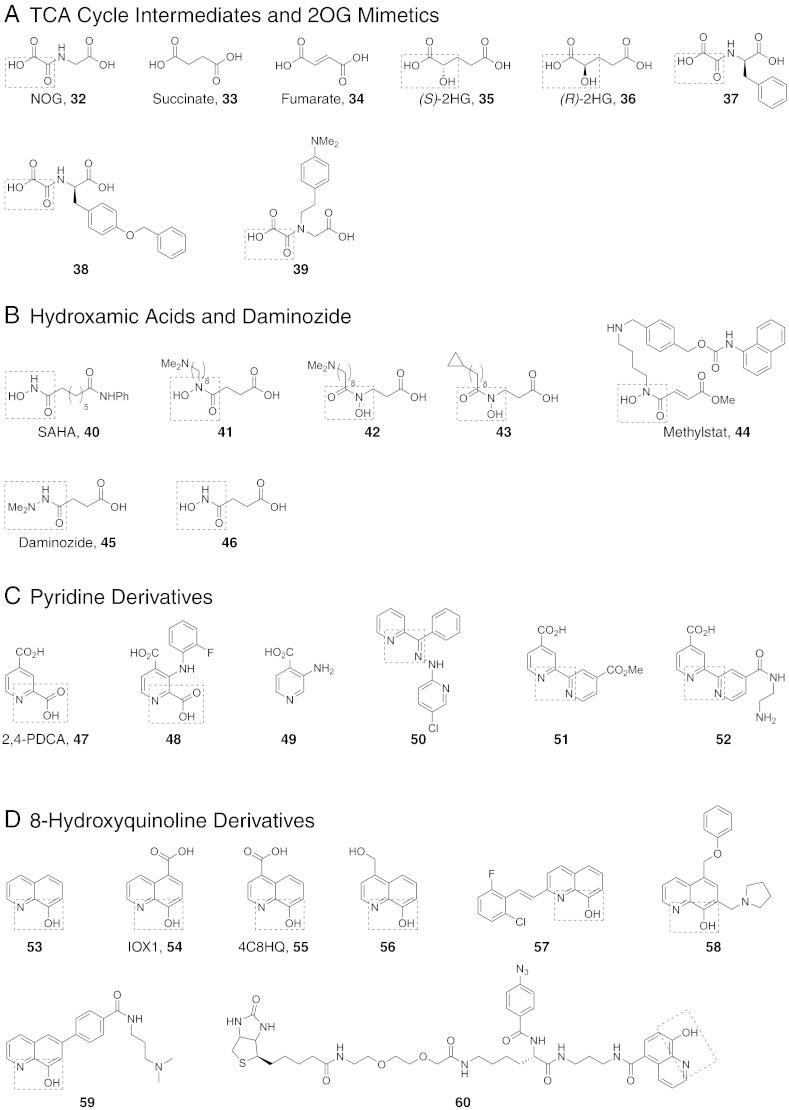
Fig. 4.
Structures of representative iron-chelating inhibitors of the JmjC KDMs. (A) Structures of tricarboxylic acid (TCA) cycle intermediates and 2OG mimetics. 2OG is a co-substrate of the 2OG oxygenases. Some 2OG oxygenases are inhibited by succinate (33, a co-product of catalysis), fumarate (34), and 2-hydroxyglutarate (35 and 36). Levels of these small molecules can be substantially increased in some tumour cells. N-Oxalylglycine (NOG, 32) is a close isostere of 2OG. C-α derivatisation of NOG can confer selectivity for different 2OG oxygenase subfamilies. (B) Structures of hydroxamic acid-containing inhibitors and Daminozide (45). Hydroxamic acids are established metal chelators and with appropriate functionalisation can be potent and selective 2OG oxygenase inhibitors. Examples include the JmjC KDM inhibitor Methylstat (44), and SAHA (40), which is a clinically used inhibitor of histone deacetylases that also inhibits JmjC KDMs in vitro. Daminozide (45), a small achiral hydrazide, inhibits the KDM2/7 subfamily of JmjC KDMs. (C) Examples of pyridine-based KDM inhibitors. Pyridine-2,4-dicarboxylic acid (2,4-PDCA, 47) is a broad-spectrum 2OG oxygenase inhibitor that chelates active site-bound iron via its pyridyl nitrogen and 2-carboxylate. KDM4-selective derivatives of 2,4-PDCA have been prepared via substitution at the 3-position (e.g. 48). Recently, pyridine-containing fragments without a 2-carboxylate group (e.g. 49) have also been reported to inhibit JmjC KDMs via monodentate iron chelation. Other pyridine-containing inhibitors include bipyridyl compounds (e.g. 51 and 52), which chelate iron via both pyridyl nitrogens, and the pyridylhydrazone 50, which was recently identified from a high-throughput screen. (D) Structures of 8-hydroxyquinoline derivatives. 8-Hydroxyquinolines chelate iron in a bidentate manner via their pyridyl nitrogen and phenolic oxygen atoms. 5-Carboxy-8-hydroxyquinoline (IOX1, 54), which was identified from a high-throughput screen against KDM4E, is a broad-spectrum 2OG oxygenase inhibitor that exhibits moderate selectivity for some JmjC KDM subfamilies (KDM2/7, KDM3, KDM4, KDM6). Substitution at the 2-, 4-, 5- and 7- positions of the 8-hydroxyquinoline ring has resulted in improved selectivity for KDMs (e.g. 56-59). Crystallographic studies with KDM4A, KDM6B and the HIF asparaginyl hydroxylase FIH reveal that IOX1 can induce translocation of the active-site iron (see Fig. 5, Fig. 7). Such metal movement is not observed for 4-carboxy-8-hydroxyquinoline (4C8HQ, 55) binding to KDM4A (Fig. 5).