Figure 1.
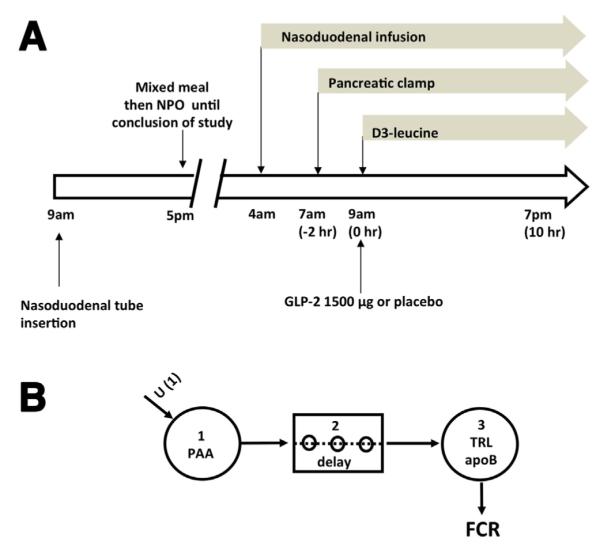
(A) Outline of lipoprotein kinetic study (study A). Volunteers had a nasoduodenal tube inserted the day before the study. After an overnight fast, a liquid mixed macronutrient formula was infused through the nasoduodenal tube for 15 hours from 4 AM on the day of the study. A pancreatic clamp (with infusion of somatostatin, insulin, glucagon, and growth hormone) was started at 7 AM. TRL kinetics were studied with a primed, constant infusion of deuterated leucine (d3-leucine) for 10 hours starting at 9 AM. At 9 AM volunteers received a subcutaneous dose of either GLP-2 (1500 ug) or placebo. NPO, Nil per oral except water. (B) Multicompartmental model for analysis of TRL apoB-100 kinetics and TRL apoB-48 placebo treatment. Infused d3-leucine enters the plasma amino acid pool (PAA) (compartment 1). After a delay (compartment 2), it is incorporated into TRL apoB (compartment 3). Enrichment time-course curves were analyzed with the multicompartmental model to derive FCR.
