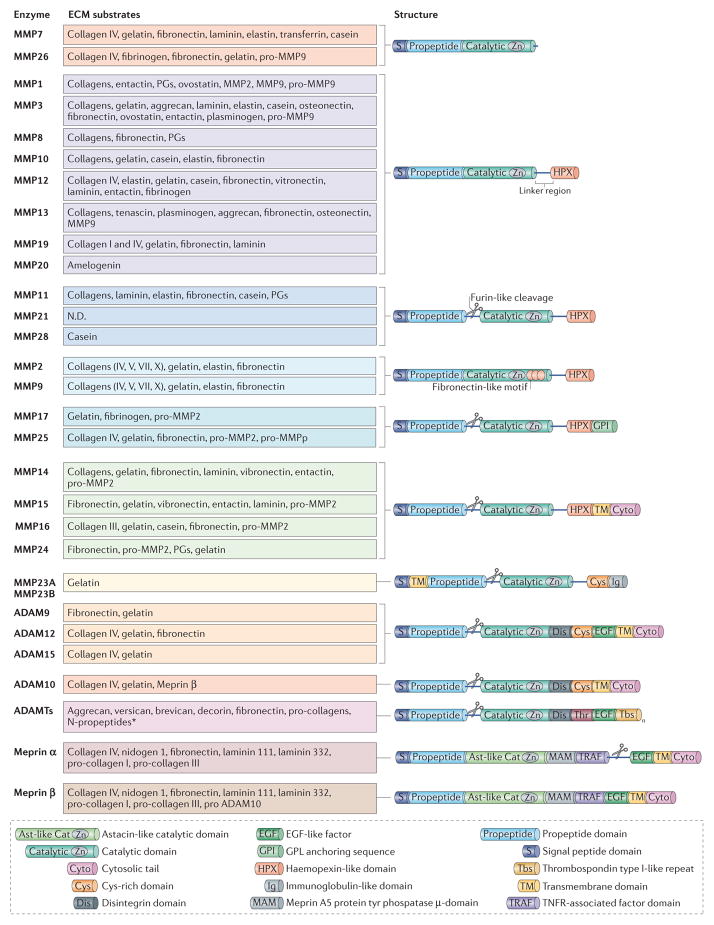
Figure 1. Structure and targets substrates of metalloproteinases.
Metalloproteinases belong to the metzincin enzyme family, which includes matrix metalloproteinases(MMPs), adamalysins (which includes ADAMs (a disintegrin and metalloproteinases) and ADAMTS (ADAMs with a thrombospondin motif)) and astacins (including meprins). They are multidomain enzymes that contain the highly conserved motif HEXXHXXGXXH (where X is any amino acid), in which three His residues chelate a zinc ion in the catalytic site. Metalloproteinases are produced either as soluble or membrane-anchored enzymes that cleave components of the extracellular matrix (ECM). MMPs are composed of several shared functional domains: signal peptide domain, propeptide domain, catalytic domain and haemopexin-like domain (except MMP7, MMP23 and MMP26). The amino-terminal signal peptide domain is required for the secretion of MMPs. The propeptide domain contains the Cys-switch motif PRCGXPD. The catalytic domain (which has proteolytic activity) contains the zinc-binding motif; the Cys residue in this motif interacts with the zinc ion that keeps pro-MMPs inactive until the propeptide domain is removed. The carboxy-terminal haemopexin-like domain, which is present in almost all MMPs, is involved in substrate specificity and in the non-proteolytic functions of MMPs160. Membrane-type MMPs (MTMMPs) such as MMP14 are anchored to the cell surface by either a transmembrane domain followed by a short cytoplasmic tail or a glycosylphosphatidylinositol (GPI) sequence. Some MMPs, including MTMMPs, MMP11, MMP17, MMP21, MMP23, MMP25 and MMP28 can be activated by the furin convertase, which cleaves the propeptide of inactive precursors in the Golgi apparatus, to release functional proteins. ADAMs are transmembrane proteins that are structurally similar to MTMMPs, except that they lack the haemopexin domain and instead have three other domains: the Cys-rich domain, the epidermal growth factor (EGF)-like repeat domain (except ADAM10 and ADAM17) and the disintegrin domain. Only ADAM9, ADAM10, ADAM12 and ADAM15 are shown, as the other ADAMs do not have known ECM protein substrates. ADAMTSs are secreted proteinases and have thrombospondin type I-like repeats in their C-terminal sequence. In addition to the metalloproteinase domains, the meprins also have an astacin-like catalytic domain (Ast-like Cat), a MAM (meprin A5 protein Tyr phosphatase) domain, a TRAF (TNFR-associated factor) domain and a C-terminal cytosolic tail. Meprin-α also contains a furin cleavage domain, cleavage of which results in the loss of the EGF-like transmembrane domain and the cytosolic domain and release of the enzyme into the extracellular space. MMP23 contains an immunoglobulin (Ig) domain that is unique among the MMPs. This Ig domain facilitates protein–protein or lipid–protein interactions similar to the haemopexin domain of other MMPs.