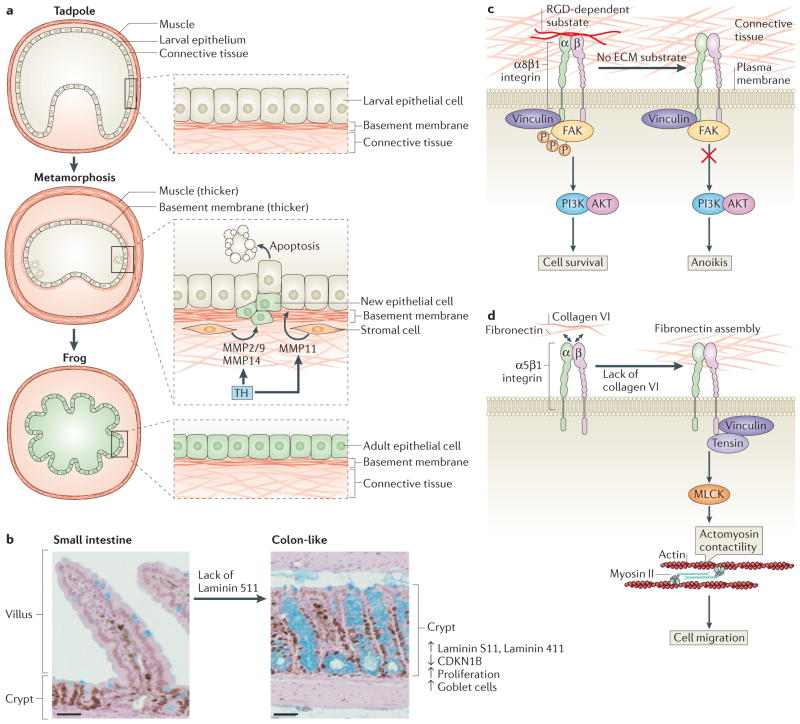
Figure 2. Extracellular matrix remodelling during intestinal development.
a | Tadpole-to-adult intestinal epithelium remodelling during Xenopus laevis morphogenesis. In the pre-metamorphosis tadpole, the small intestine consists of a single layer of larval epithelium (also known as typhlosole), connective tissue and a thin muscle layer. During metamorphosis, thyroid hormone (TH) is produced in high levels, inducing the release of matrix metalloproteinase 11 (MMP11) by stromal cells to trigger apoptosis of larval epithelial cells. At the same time, proliferating stem and progenitor cells give rise to new adult epithelial cells that replace the larval epithelium. During metamorphosis, the basement membrane and the muscle layer are thicker. The levels of other MMPs, such as MMP2 and/or MMP9 and MMP14, increase during tadpole metamorphosis after epithelial cell death, suggesting that they may have a role post-apoptosis. At the end of metamorphosis, the differentiated adult intestine becomes capable of self-renewal and forms a multiply folded epithelium, similar to the mammalian adult intestine.
b–d | Intestinal epithelium remodelling in other vertebrates. Laminin distribution during mammalian intestine development determines small intestine and colon architecture. The basement membrane of villi in the intestine is composed mainly of laminin 511 α5 chain. In mice, a lack of laminin 511 in the intestinal basement membrane leads to a compensatory deposition of colonic laminins (laminin 111 and laminin 411), which results in the transformation of the small intestinal to a tissue with a colon-like mucosal structure that shows high levels of cell proliferation, low levels of the cell cycle inhibitor cyclin-dependent kinase inhibitor B (CDKN1B), and higher numbers of goblet cells (part b). RGD-dependent substrates such as fibronectin can bind to α8β1 integrin. This anchorage prevents anoikis in undifferentiated human intestinal epithelial crypt (HIEC) cells through the recruitment of vinculin and the activation of the PI3K–AKT signalling pathway (part c). Collagen VI is produced by HIEC cells and regulates fibronectin assembly by restraining cell–fibronectin interactions, which influences cell functions such as migration. A lack of collagen VI leads to recruitment of tensin at the fibrillar adhesion points via the activation of myosin light chain kinases (MLCKs), which mediate actomyosin contractility, extensive fibrillogenesis and cell migration (part d). FAK, focal adhesion kinase. Figure part a modified from Establishment of intestinal stem cell niche during amphibian metamorphosis. Curr. Top. Dev. Biol Volume 103. Chapter 11. Pages 305–327. 2013. With permission from Elsevier.161