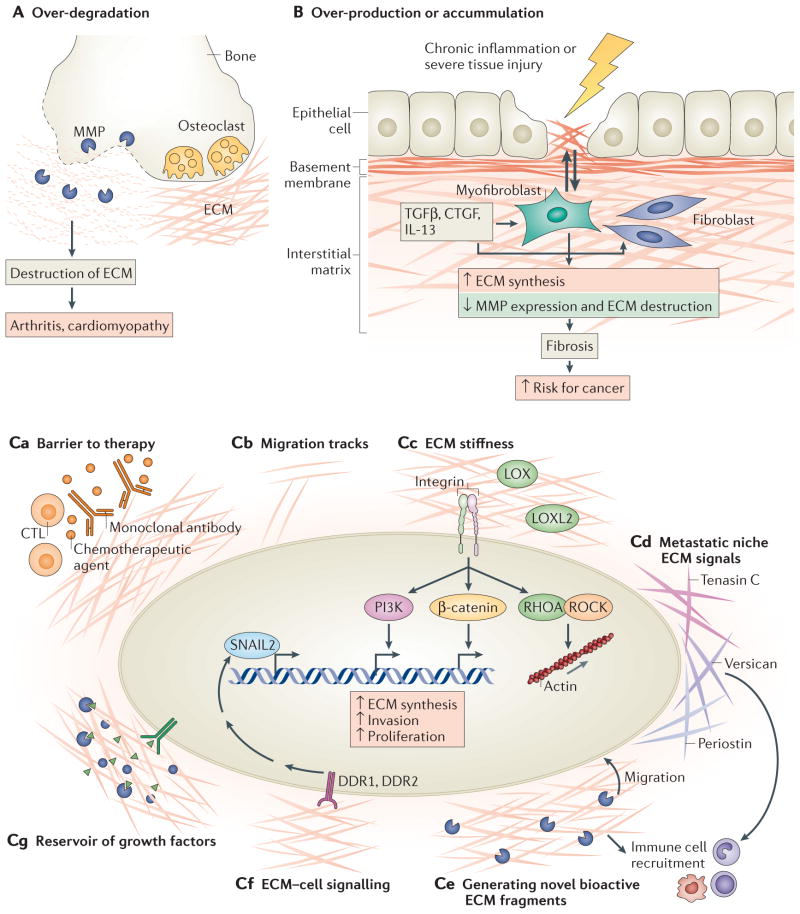
Figure 4. Aberrant extracellular matrix remodelling leads to numerous human diseases.
A | Over-degradation of the extracellular matrix (ECM), mediated primarily by matrix metalloproteinases (MMPs) and ADAMTS (ADAMs with a thrombospondin motif), results in osteoarthritis and increased breakdown of the connective tissue. B | As a result of chronic inflammation or tissue injury, transforming growth factor-β (TGFβ), connective tissue growth factor (CTGF), interleukin-13 (IL-13) and other factors stimulate fibroblasts and myofibroblasts (the main ECM producers) to produce more ECM, resulting in pathological fibrosis. The excess ECM further stimulates fibroblasts to continue making ECM, forming a positive feedback loop. Fibrosis is a major risk factor for developing cancer, including hepatocellular carcinoma and breast cancer. C | The ECM contributes to cancer pathogenesis by several mechanisms: functioning as a barrier to chemotherapy, to monoclonal antibodies such as cetuximab and to immune therapy mediated, for example, by cytotoxic T cells (CTLs) (part Ca); forming migration track ‘highways’ that regulate the interaction of immune cells with cancer cells (part Cb); stimulating integrin signalling through increased ECM stiffness, which promotes ECM synthesis, invasion and proliferation (lysyl oxidase (LOX) and LOX-like 2 (LOXL2) are enzymes that crosslink collagen and are the main enzymes responsible for increasing ECM stiffness) (part Cc); forming a niche for new metastatic cells and providing survival and proliferative signals (part Cd); generating novel bioactive ECM fragments from native ECM chains, and stimulating cell migration or immune cell recruitment (part Ce); activating cell–ECM receptors such as discoidin domain-containing receptor 1 (DDR1) and DDR2, which bind directly to collagen and regulate transcriptional pathways to increase MMP and epithelial–mesenchymal transition (EMT) marker expression (part Cf); and sequestering growth factors that can be released by proteolytic cleavage, which then diffuse to bind receptors to stimulate cell growth, EMT or angiogenesis (part Cg).