Abstract
IMPORTANCE
Postoperative hypocalcemia is common after total thyroidectomy, and perioperative monitoring of serum calcium levels is arguably the primary reason for overnight hospitalization. Confidently predicting which patients will not develop significant hypocalcemia may allow for a safe earlier discharge.
OBJECTIVE
To examine associations of patient characteristics with hypocalcemia, duration of hospitalization, and postoperative intact parathyroid hormone (IPTH) level after total thyroidectomy.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective study of consecutive patients who underwent total thyroidectomy by a single high-volume surgeon between February 1, 2010, and November 30, 2012. Postoperative serum 25-hydroxyvitamin D (vitamin D), calcium, and IPTH levels were tested within 6 to 8 hours after surgery. Mild hypocalcemia was defined as any postoperative serum calcium level of less than 8.4 to 8.0 mg/dL. Significant hypocalcemia was defined as any postoperative serum calcium level of less than 8.0 mg/dL or the development of hypocalcemia-related symptoms.
INTERVENTIONS
Total thyroidectomy.
MAIN OUTCOMES AND MEASURES
Associations of patient demographic and clinical characteristics and laboratory values with postoperative mild and significant hypocalcemia were examined using univariate analysis, and independent predictors of hypocalcemia, duration of hospitalization, and IPTH level were determined using multivariate analysis.
RESULTS
Overall, 304 total thyroidectomies were performed. Mild and significant hypocalcemia occurred in 68 (22.4%) and 91 (29.9%) patients, respectively, of which the majority were female (P = .003). The development of significant hypocalcemia was associated with postoperative IPTH level (P < .001). On multivariate analysis, males had a decreased risk of developing mild (odds ratio, 0.37 [95% CI, 0.16–0.85]) and significant (odds ratio, 0.57 [95% CI, 0.09–0.78]) hypocalcemia. Every 10-pg/mL increase in postoperative IPTH level predicted a 43% decreased risk of significant hypocalcemia (P < .001) and an 18% decreased risk of hospitalization beyond 24 hours (P = .03). Presence of malignant neoplasm carried a 27% risk of mild hypocalcemia (P = .02). There was a progressively increasing risk of lower IPTH levels for each parathyroid gland inadvertently resected or autotransplanted. Male sex and African American race were independently predictive of higher IPTH levels.
CONCLUSIONS AND RELEVANCE
Low postoperative IPTH level, female sex, and presence of malignant neoplasm are all significant, independent predictors of hypocalcemia after total thyroidectomy. Clinicians should consider these variables when deciding how to best manage or prevent postoperative hypocalcemia.
Because of the current strain on the US health care system, increasing emphasis is being placed on outpatient management of conditions that necessitate surgical procedures. In the modern era, a paradigm shift has taken place in which an increasing number of operations that were previously managed with postoperative hospitalization are now commonly accomplished as outpatient procedures.1–3 Similarly, total thyroidectomy is now being performed as a short-stay or even an outpatient procedure at some medical centers.4 However, this shift in management has occurred in the absence of consensus and evidence-based parameters for defining the population of patients eligible to undergo outpatient total thyroidectomy.5
Defining a framework for safe outpatient thyroidectomy is crucial, especially given that the complication rates following thyroidectomy are not insignificant (7.4%–13.8%).6,7 Hypocalcemia after thyroidectomy is the most common complication, with the reported incidence of transient and permanent hypocalcemia ranging from 3% to 52% and 0.4% to 13%, respectively.8,9 Various strategies for diagnosing and managing postoperative hypocalcemia have been used. The traditional approach of 2-day hospitalization and monitoring of serum calcium levels after surgery is still being used by many institutions worldwide because the nadir of hypocalcemia typically occurs within 48 hours after surgery.10,11 We agree that it is important to observe patients in the initial postoperative hours for hemorrhage and airway obstruction that may necessitate an urgent return to the operating room; however, calcium monitoring with hospitalization beyond 24 hours, in the absence of apparent perioperative complications, is often unnecessary because patients typically experience only mild postoperative pain and rapidly return to baseline daily functionality.
The routine use of postoperative oral calcium and/or vitamin D supplementation has been advocated by some surgeons to minimize the incidence of hypocalcemia and shorten hospital stays. Such routine use is particularly common in the outpatient or short-stay setting, where there is limited time available to correct hypocalcemia once it is discovered. Others have advocated sending patients home with prescriptions for elemental calcium supplementation to be filled if symptoms of hypocalcemia develop.12,13 More recently, with the aim of finding an earlier predictor for hypocalcemia, the short half-life of the parathyroid hormone has led to increased interest in postoperative intact parathyroid hormone (IPTH) as an early marker of hypocalcemia.11,14–19 However, the routine measurement of IPTH to assess the risk of postoperative hypocalcemia has yet to become accepted as standard practice. The variability in assays, timing of measurements, and cutoff levels makes comparisons between studies difficult.20
It is crucial to identify the most reliable early determinants of hypocalcemia to help surgeons distinguish those patients who are at low risk for developing hypocalcemia from those who need calcium supplementation therapy and inpatient observation. The ability to discriminate between these groups may allow for an up to 50% cost reduction compared with traditional postoperative hospital stays.12 The objective of the present study was to identify independent risk factors for the development of postoperative hypocalcemia and to identify subgroups of patients at particularly high or low risk. The ability to consistently identify patients who are at risk for developing hypocalcemia may allow surgeons to confidently select patients who can undergo these procedures on an out-patient or short-stay basis.
Methods
Data Source
This retrospective study was approved by the institutional review board of Johns Hopkins University School of Medicine, and informed consent was waived. Data were collected from the medical records of all patients who underwent a total thyroidectomy with or without central neck dissection between February 1, 2010, and November 30, 2012. All procedures were performed by a single surgeon (R.P.T.) at a university-based tertiary medical center. Using Current Procedural Terminology codes, we identified all patients who underwent total thyroidectomy (60240), substernal bilateral thyroidectomy with or without sternal split (60270, 60271), and total thyroidectomy with limited (central) neck dissection (60252). A compartment-oriented neck dissection was performed for patients with clinically apparent lymph node metastases. A prophylactic compartment-oriented neck dissection was only performed for patients with medullary thyroid cancer. Patients undergoing intentional parathyroidectomy for primary hyperparathyroidism at the time of thyroidectomy were excluded.
Assessment of Postoperative Hypocalcemia
Routine serum calcium, IPTH (reference range, 10–65 pg/mL; to convert to nanograms per liter, multiply by 1.0), and 25-hydroxyvitamin D (vitamin D) levels were measured within 6 to 8 hours after completion of the operation. Patients were considered to have postoperative mild hypocalcemia if they had at least 1 postoperative serum calcium level of less than 8.4 but no value of less than 8.0 mg/dL (reference range, 8.4–10.2 mg/dL; to convert to millimoles per liter, multiply by 0.25). Patients were considered to have postoperative significant hypocalcemia if they had a postoperative serum calcium level of less than 8.0 mg/dL on at least 1 laboratory test or if they developed hypocalcemia-related symptoms (diffuse perioral or fingertip paresthesias or numbness, tetany, or a newly positive Chvostek sign).
For patients who developed hypocalcemia, oral calcium carbonate supplementation was initiated or increased (from preoperative dosage) with or without calcitriol, and intravenous calcium gluconate was administered for symptoms. Any new calcium or calcitriol supplementation postoperatively, or increase from preoperative dose, was considered calcium supplementation therapy. Oral calcium supplementation and, when indicated, calcitriol were continued until postoperative follow-up. The decision to continue or discontinue use of these medications was based on physical examination, laboratory results, and clinical judgment of the surgeon (R.P.T.) at postoperative encounters. All patients were eventually able to discontinue use of calcitriol. Patients were categorized as permanently hypocalcemic when calcium supplementation therapy was required to maintain normocalcemia beyond 6 months following surgery.
Statistical Analysis
Data analysis was performed using Stata, version 11 (StataCorp). On univariate analysis, continuous variables were compared using 1-way analysis of variance, and categorical variables were compared using the χ2 or Fisher exact test. Multivariate logistic regression was used to determine independent predictors of hypocalcemia, duration of hospitalization, and postoperative IPTH level. All reported P values are 2 sided, and a level of .05 was used to evaluate statistical significance.
Results
A total of 304 eligible thyroid procedures during the study period were identified. Postoperative mild hypocalcemia occurred in 68 patients (22.4%). Significant postoperative hypocalcemia occurred in 91 patients (29.9%); of those, 87 (96%) had at least 1 serum calcium level of less than 8.0 mg/dL and 22 (24%) developed hypocalcemia-related symptoms. Postoperative permanent hypocalcemia was encountered in only 2 patients (0.7%); both were female and developed significant immediate postoperative hypocalcemia. As summarized in Table 1, 76% and 79% of patients who developed postoperative mild and significant hypocalcemia, respectively, were female (P = .003). In addition, we found that patients who developed postoperative mild and significant hypocalcemia were, respectively, a mean of 1 and 5 years younger than patients who did not (P = .02).
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Variable | No. (%)a | P Value | ||
|---|---|---|---|---|
| Normocalcemia (n = 145) | Mild Hypocalcemia (n = 68) | Significant Hypocalcemia (n = 91) | ||
| Age, mean (SD), y | 51.5 (15.6) | 50.7 (16.1) | 45.9 (14.1) | .02 |
| Female sex | 87 (60) | 52 (76) | 72 (79) | .003 |
| Race | ||||
| White | 107 (74) | 49 (72) | 70 (77) | .049 |
| African American | 22 (15) | 6 (9) | 6 (7) | |
| Asian | 2 (1) | 7 (10) | 5 (5) | |
| Other | 14 (10) | 6 (9) | 10 (11) | |
| Specific thyroid diagnosis | ||||
| Papillary thyroid carcinoma | 72 (50) | 25 (37) | 46 (51) | …b |
| Graves disease | 7 (5) | 4 (6) | 8 (9) | |
| Hashimoto thyroiditis | 11 (8) | 4 (6) | 5 (6) | |
| Goiter | 33 (23) | 23 (34) | 22 (24) | |
| Nodule | 14 (10) | 6 (9) | 4 (4) | |
| Follicular thyroid carcinoma | 1 (1) | 2 (3) | 0 | |
| Hurthle cell carcinoma | 1 (1) | 2 (3) | 0 | |
| Medullary thyroid carcinoma | 4 (3) | 0 | 5 (6) | |
| Anaplastic thyroid carcinoma | 1 (1) | 1 (1) | 0 | |
| Insular cell thyroid carcinoma | 0 | 1 (1) | 0 | |
| Type of thyroidectomy | ||||
| Total | 94 (65) | 41 (60) | 52 (57) | .10 |
| Total + CND | 26 (18) | 15 (22) | 26 (29) | |
| Substernal, cervical approach | 24 (17) | 9 (13) | 9 (10) | |
| Substernal, sternal split | 0 | 2 (3) | 3 (3) | |
| Substernal, cervical approach + CND | 1 (1) | 1 (1) | 0 | |
| Substernal, sternal split + CND | 0 | 0 | 1 (1) | |
Abbreviation: CND, central neck dissection.
Percentages may not sum to 100% because of rounding.
Unable to determine a reliable P value for comparison between subgroups because of the large number of categories.
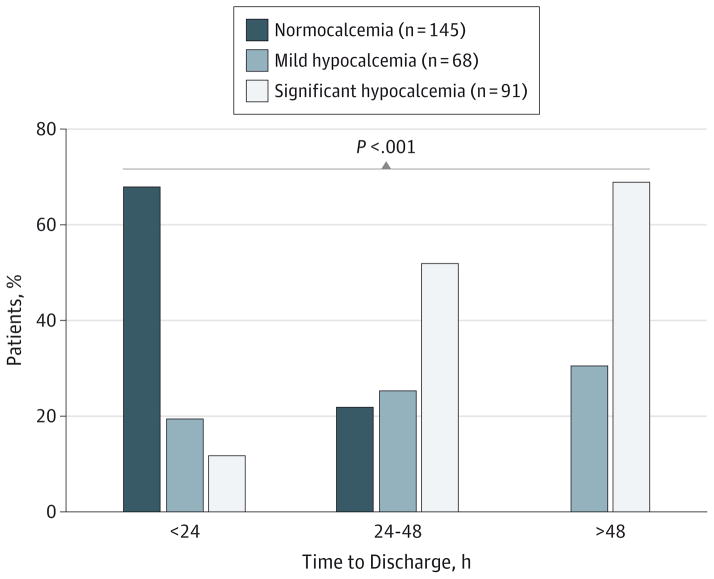
Overall, the surgical indications for thyroidectomy were similar between patients who developed postoperative mild and significant hypocalcemia and those who did not (P = .37). Of the 163 patients who underwent thyroidectomy for a suspected malignant neoplasm, 30 (18.4%) and 56 (34.4%) were found to have mild and significant hypocalcemia, respectively. Of the 7 patients with hypothyroidism who underwent thyroidectomy for benign disease, 2 (29%) were found to have mild hypocalcemia, and 2 (29%) were found to have significant hypocalcemia. Of the 99 euthyroid patients who underwent thyroidectomy for benign disease, 28 (28%) and 22 (22%) were found to have mild and significant hypocalcemia, respectively. Of the 35 patients with hyperthyroidism and benign disease or those with disease related directly to their hyperthyroid state (eg, Graves disease, chronic lymphocytic thyroiditis) who underwent thyroidectomy, 8(23%) and 11 (31%) were found to have mild and significant hypocalcemia, respectively. Table 1 summarizes the differences in thyroid diagnosis and extent of surgery performed between hypocalcemic and normocalcemic patients. Regarding duration of postoperative hospitalization, 82% of the normocalcemic patients were discharged within 24 hours after surgery. Only 20% and 12% of the patients discharged within 24 hours had mild and significant hypocalcemia, respectively (P < .001) (Figure 1).
Figure 1. Proportions of Patients With Hypocalcemia vs Normocalcemia by Duration of Hospital Stay.
Of the patients discharged within 24 hours after surgery, 68%, 20%, and 12% had normocalcemia, mild hypocalcemia, and significant hypocalcemia, respectively. Of the patients discharged between 24 and 48 hours after surgery, 22%, 26%, and 52% had normocalcemia, mild hypocalcemia, and significant hypocalcemia, respectively. Of the patients discharged beyond 48 hours after surgery, 31% and 69% had mild and significant hypocalcemia, respectively.
On univariate analysis, we found that every 10-year increase in age was associated with a 20% decreased risk of postoperative significant hypocalcemia (odds ratio [OR], 0.80 [95% CI, 0.68–0.94]; P = .006). Age was not a predictor of mild hypocalcemia (OR, 0.97 [95% CI, 0.81–1.17]; P = .75). Males had a 54% and 50% decreased risk of developing mild and significant hypocalcemia compared with females (OR, 0.46 [95% CI, 0.24–0.89]; P = .02 and OR, 0.50 [95% CI, 0.28–0.88]; P = .02, respectively). Asians had a 7.64 odds (95% CI, 1.53–38.14) of developing mild hypocalcemia compared with whites (P = .01). Although Asian race did not predict significant hypocalcemia (OR, 1.23 [95% CI, 0.40–3.83]; P = .71), they had a 5.59 odds (95% CI, 1.22–25.55) of having a postoperative calcium level of less than 8.4 mg/dL compared with whites (P = .03).
Undergoing central neck dissection did not predict mild (OR, 1.34 [95% CI, 0.67–2.71]; P = .41) or significant (OR, 1.67 [95% CI, 0.95–2.92]; P = .07) hypocalcemia. Intraoperative parathyroid gland autotransplantation or inadvertent removal did not predict mild hypocalcemia (OR, 0.96 [95% CI, 0.54–1.73]; P = .90). Conversely, if any parathyroid gland was autotransplanted or inadvertently removed intraoperatively, there was a 70% increased risk of developing significant hypocalcemia (OR, 1.70 [95% CI, 1.04–2.79]; P = .04), and for each individual parathyroid gland autotransplanted or inadvertently removed, there was a 60% increased risk of significant hypocalcemia (OR, 1.60 [95% CI, 1.18–2.17]; P = .003).
Postoperative serum vitamin D levels were not predictive of postoperative mild or significant hypocalcemia (P = .46 and .69, respectively). As seen in Table 2, there was a progressive decreased risk of significant hypocalcemia with increasing postoperative IPTH levels. There was a 20%, 14%, and 5% risk of significant hypocalcemia, respectively, for patients with IPTH levels of 10 to 19, 20 to 29, and at least 30 mg/dL compared with those patients with an IPTH level of less than 10 mg/dL (P < .001 for all). This finding was consistent on confirmatory analysis treating IPTH as a continuous variable; for every 10-pg/mL increase in postoperative IPTH, there was a 46% decrease in the risk of developing significant hypocalcemia (OR, 0.54 [95% CI, 0.44–0.67]; P < .001).
Table 2.
Predictors of Postoperative Mild and Significant Hypocalcemia on Univariate Analysis
| Predictor Variable of Mild Hypocalcemia | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Postoperative IPTH level, pg/mL | ||
| <10 | 1 [Reference] | |
| 10–19 | 0.67 (0.18–2.60) | .57 |
| 20–29 | 0.23 (0.06–0.92) | .04 |
| ≥30 | 0.39 (0.11–1.36) | .14 |
| Postoperative IPTH level, pg/mL (continuous) | 1.01 (0.87–1.16) | .94 |
| Postoperative vitamin D level, ng/mL | ||
| <20 | 1 [Reference] | |
| 20–39 | 0.57 (0.21–1.57) | .28 |
| ≥40 | 0.43 (0.15–1.24) | .12 |
| Postoperative vitamin D level, ng/mL (continuous) | 0.93 (0.78–1.12) | .46 |
| Predictor Variable of Significant Hypocalcemia | ||
| Postoperative IPTH level, pg/mL | ||
| <10 | 1 [Reference] | |
| 10–19 | 0.20 (0.09–0.48) | <.001 |
| 20–29 | 0.14 (0.06–0.33) | <.001 |
| ≥30 | 0.05 (0.02–0.12) | <.001 |
| Postoperative IPTH level, pg/mL (continuous) | 0.54 (0.44–0.67) | <.001 |
| Postoperative vitamin D level, ng/mL | ||
| <20 | 1 [Reference] | |
| 20–39 | 1.48 (0.55–3.97) | .44 |
| ≥40 | 1.71 (0.62–4.71) | .30 |
| Postoperative vitamin D level, ng/mL (continuous) | 1.03 (0.89–1.20) | .69 |
Abbreviation: IPTH, intact parathyroid hormone.
SI conversion factors: To convert IPTH level to nanograms per liter, multiply by 1.0; to convert vitamin D level to nanomoles per liter, multiply by 2.496.
On multivariate analysis, after controlling for age, sex, race, indication for thyroidectomy, diagnosis, type of procedure, number of parathyroid glands removed, and postoperative IPTH and vitamin D levels, we found that males had a 63% and 56% decreased risk of developing postoperative mild and significant hypocalcemia, respectively (Table 3). Mild hypocalcemia was also predicted by the presence of thyroid malignant neoplasm (OR, 0.27 [95% CI, 0.09–0.78]; P = .02). In addition, there was a progressively increasing risk of having a lower IPTH level for each parathyroid gland resected or autotransplanted. A higher IPTH level (≥20 pg/mL) was predicted by male sex (OR, 1.98 [95% CI, 1.01–3.91]; P = .047) and African American race (OR, 3.41 [95% CI, 1.12–10.40]; P = .03) compared with females and other races, respectively. An IPTH level of at least 30 pg/mL was predicted by African American race (OR, 4.20 [95% CI, 1.60–10.98]; P = .003). For every 10-pg/mL increase in the postoperative IPTH level, there was a 43% decreased risk of significant hypocalcemia (OR, 0.57 [95% CI, 0.45–0.72]; P < .001) and an 18% decreased risk of hospitalization longer than 24 hours (OR, 0.82 [95% CI, 0.68–0.98]; P = .03).
Table 3.
Multivariate Logistic Regression Analysis for Postoperative Outcomes After Thyroid Surgery
| Outcome Variable | Predictor Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| Postoperative mild hypocalcemia | Male sex | 0.37 (0.16–0.85) | .02 |
| Malignant neoplasm | 0.27 (0.09–0.78) | .02 | |
| Postoperative significant hypocalcemia | Postoperative IPTH level, per 10 pg/mL | 0.57 (0.45–0.72) | <.001 |
| Male sex | 0.44 (0.21–0.92) | .03 | |
| Hospitalization >24 h | Mild hypocalcemia | 5.31 (2.34–12.03) | <.001 |
| Significant hypocalcemia | 13.56 (5.90–31.21) | <.001 | |
| Postoperative IPTH level, per 10 pg/mL | 0.82 (0.68–0.98) | .03 | |
| Hashimoto thyroiditis | 0.16 (0.04–0.67) | .01 | |
| Postoperative IPTH level ≥10 pg/mL | No. of parathyroid glands removed | 0.46 (0.29–0.74) | .001 |
| Postoperative IPTH level ≥20 pg/mL | Male sex | 1.98 (1.01–3.91) | .047 |
| African American race | 3.41 (1.12–10.40) | .03 | |
| Central neck dissection | 0.43 (0.19–0.94) | .04 | |
| No. of parathyroid glands removed | 0.62 (0.42–0.91) | .02 | |
| Postoperative IPTH level ≥30 pg/mL | African American race | 4.20 (1.60–10.98) | .003 |
Abbreviation: IPTH, intact parathyroid hormone.
SI conversion factor: To convert IPTH level to nanograms per liter, multiply by 1.0.
Of all the male patients with a postoperative IPTH level of at least 30 pg/mL, only 4 (14%) and 1 (3%) developed postoperative mild and significant hypocalcemia (P = .03 and .11) and 3 (19%) and 0 with IPTH of at least 40 pg/mL (P = .22 and .18), respectively. Only 10 (20%) and 6 (11%) males with an IPTH level of 20 pg/mL or more developed mild and significant hypocalcemia (P = .08 and .049), respectively. Conversely, 25 (39%) and 12 (16%) female patients with an IPTH of at least 30 pg/mL developed mild and significant hypocalcemia (P = .03 and .11), and 5 (71%) and 24 (77%) female patients with an IPTH of less than 10 pg/mL had postoperative mild and significant hypocalcemia (P = .24 and .71), respectively.
Discussion
Various factors have been reported to influence the risk of postoperative hypocalcemia, including biochemical, patient-related, disease-related, and surgical factors.10,21,22 These factors may help to identify the most reliable markers of early hypocalcemia, helping surgeons to risk stratify patients for short-stay or outpatient total thyroidectomy.
Patient-Related and Disease-Related Factors
The literature is divided as to whether age is a risk factor for hypocalcemia after thyroidectomy. A small number of studies have found hypocalcemia to be associated with advanced age,15,23–25 whereas others have reported an association with younger age.26–28 A recent meta-analysis by Edafe et al20 evaluated age as a predictor of hypocalcemia in 2576 patients and found no significant difference in mean age between patients with hypocalcemia and those without. Conversely, in our study we observed that patients who developed postoperative mild and significant hypocalcemia were a mean of 1 and 5 years younger than patients who did not (P = .02). We also found that every 10-year increase in age was associated with a 20% decreased risk in developing postoperative significant hypocalcemia (P = .006) on univariate analysis. However, in the present study, on multivariate analysis, after adjustment for demographic and clinical characteristics and laboratory values, age was found not to be an independent predictor of hypocalcemia.
The literature also contains conflicting reports with regard to associations with sex. In some studies, sex has not been shown to affect calcium homeostasis postoperatively.29,30 In contrast, a large body of literature reports that female sex is associated with a significantly higher incidence of hypocalcemia compared with males.20,24,27,28,31–34 In our study, 76% and 79% of patients who developed postoperative mild and significant hypocalcemia were female (P = .003), respectively, and male sex was found to be independently protective against postoperative hypocalcemia.
Few authors discuss the association of race with the development of postoperative hypocalcemia. A study by Burge et al35 found that race is not associated with postoperative hypocalcemia. On multivariate analysis, our study also did not find an association with race in predicting mild or significant hypocalcemia. Conversely, African American race did independently predict a higher (≥20 pg/mL) IPTH level compared with other races.
The positive association of Graves disease with hypocalcemia has been reported and may be due to increased bone turnover or increased vascularity of the thyroid gland, rendering these operations more difficult than others.36 Zambudio et al37 and McHenry et al38 conducted multivariate logistic regression analysis and found hyperthyroidism to be an independent predictor of postoperative hypocalcemia. Furthermore, Thomusch et al31 found that a preoperative diagnosis of Graves disease was associated with hypocalcemia after controlling for potential confounders. In our study, we did not find a positive association of Graves disease with either mild or significant hypocalcemia. Furthermore, we did not observe a significantly higher incidence of hypocalcemia in patients with hyperthyroidism when compared with euthyroid patients (P = .37), and hyperthyroidism and hypothyroidism were not independently associated with postoperative mild or significant hypocalcemia on multivariate analysis.
Other potential disease-related predictors of hypocalcemia include thyroid malignant neoplasm.35 In our study, of all the patients who underwent thyroidectomy without central neck dissection for as suspected thyroid malignant neoplasm (n = 93), 14 (15%) developed mild hypocalcemia and 29 (31%) developed significant hypocalcemia. Of patients who underwent thyroidectomy with a central neck dissection for thyroid malignant neoplasm (n = 70), 16 (23%) developed mild hypocalcemia and 27 (39%) developed significant hypocalcemia. On multivariate analysis, after demographic and clinical characteristics were controlled for, thyroid malignant neoplasm independently predicted mild hypocalcemia (P = .02).
Biochemical Factors
Perioperative IPTH is currently the most emphasized biomarker of impending postoperative hypocalcemia.11,14–19,39–41 In the systematic review and meta-analysis by Edafe et al,20 a low postoperative IPTH level (defined as less than 6–35 pg/mL) between 1 hour and 1 day after thyroidectomy had a high sensitivity (69%–100%) and specificity (81%–100%) for predicting postoperative hypocalcemia. A post operative decline in IPTH of more than 38% to 88% compared with preoperative levels was also found to have a sensitivity ranging from 70% to 100% in predicting postoperative hypocalcemia. Consistent with these reports, we found that the postoperative IPTH level was independently associated with the development of postoperative significant hypocalcemia but not mild hypocalcemia. On multivariate analysis, each 10-pg/mL increase in the IPTH level was associated with a 43% decreased risk of significant hypocalcemia (P < .001). In addition, each 10-pg/mL increase in IPTH was associated with an 18% decreased risk of hospitalization longer than 24 hours (P = .03). Generally, a low IPTH level predicts patients at risk of hypocalcemia and a mid-high normal level excludes permanent hypoparathyroidism.20 In our study, only 14% and 3% of males who had a 30 pg/mL or greater IPTH level developed postoperative mild and significant hypocalcemia (19% and 0% with IPTH ≥40 pg/mL), respectively. Conversely, 39% and 16% of females with an IPTH value of 30 pg/mL or greater developed mild and significant hypocalcemia, respectively. Also, despite vitamin D deficiency being common inpatients undergoing thyroidectomy, our results do not suggest that this increases the rate of hypocalcemia. Given that the primary effect of vitamin D is to enhance the absorption of calcium from the gastrointestinal tract and is not directly correlated with calcium levels or calcium regulation, this is not surprising. Intact parathyroid glands will maintain normal serum calcium levels as long as there is calcium available in bone that can be mobilized. Therefore, postoperative evaluation of serum vitamin D levels is unlikely to aid in the assessment of postoperative hypocalcemia risk.
Surgical Factors
Trauma, devascularization, or inadvertent excision of parathyroid glands can impair parathyroid function following thyroid surgery. Although the etiology of postoperative hypocalcemia seems to be multifactorial, reports have found the most important factor to be iatrogenic surgical trauma to the parathyroid glands, which is further exacerbated by the extent of surgical dissection.10,42 A greater extent of thyroid and central neck surgery creates not only a higher risk for accidental excision of parathyroid glands but also endangers the arterial and venous vasculature of the parathyroid glands. In our study, central neck dissection did not predict mild or significant hypocalcemia; however, a central neck dissection procedure was independently associated with a 57% decreased risk of having a postoperative IPTH level of at least 20 pg/mL.
Intraoperatively, if a parathyroid gland is excised accidentally or is devascularized, it has been the standard of care to autotransplant the gland in situ.31,41 In our series, parathyroid gland autotransplantation occurred more frequently in the hypocalcemic groups(P = .006). This may be partially explained by the fact that successful revascularization of the autotransplanted gland may take as much as 8 weeks to occur; mean while, the patient may develop transient hypocalcemia. Nonetheless, the number of parathyroid glands removed during thyroidectomy did not appear to be a major determinant of the outcome. Individual parathyroid gland removal or autotransplantation did not predict mild or significant hypocalcemia postoperatively, after adjustment for potential confounders. Moreover, it has been shown that postoperative hypocalcemia is dependent on the surgeon’s experience and the operative technique used.6,7 In this study, all operations were performed by a single high-volume thyroid surgeon (R.P.T.), and the surgical technique was uniform in all cases.
Routine administration of oral calcium and vitamin D supplements has been advocated by some surgeons to minimize the incidence of significant hypocalcemia and to increase the likelihood of early hospital discharge after total thyroidectomy.5,43 We prefer to educate patients about hypocalcemia symptoms and personalize the postoperative management of hypocalcemia according to the individual risk profile of each patient. The reason for not administering routine calcium supplementation to all our patients is that the morbidity of high-dose calcium supplementation is not negligible.44 Symptoms of gastritis and constipation are frequently reported by patients. The possibility of developing a temporary state of hypercalcemia is also a concern. The strong binding property of calcium carbonate may alter the therapeutic effects of other drugs taken by the patient,45 and possibly levothyroxine sodium. It may also alter a drug’s action by changing the stomachacidity. 44 Moreover, the potential adverse events of routine supplementation, and the need for follow-up to discontinue these supplements, represent an increase in costs that have not been considered in the analysis of cost-effectiveness studies of routine administration.43
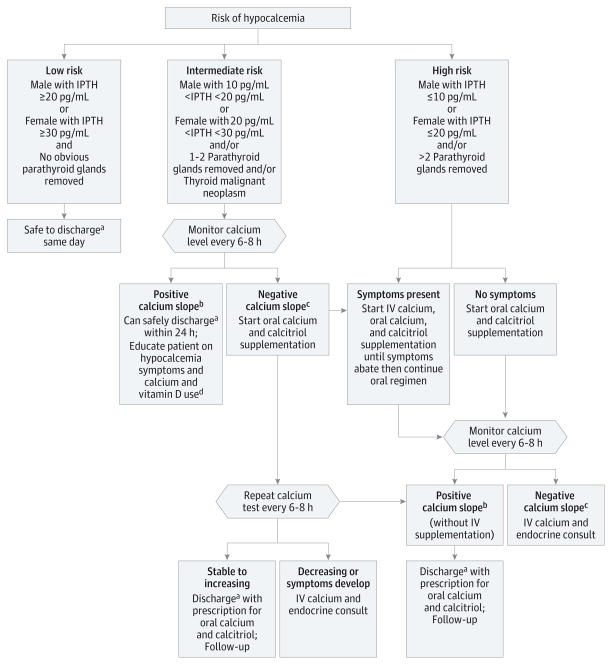
The information obtained from this study was used to create a risk stratification scheme for developing and managing postoperative hypocalcemia that we have implemented in our practice (Figure 2). We incorporated the predictors of hypocalcemia identified in this study into our previous management protocol to create a personalized algorithm for the safe management and discharge of patients after total thyroidectomy.46 The final points on the algorithm were the following recommended actions: Discharge the patient on the day of surgery if all other same-day discharge criteria are met; monitor calcium levels twice a day until deemed safe for discharge; and start oral or intravenous calcium and calcitriol supplementation depending on clinical findings.
Figure 2. Clinical Algorithm for Management of Patients at Low, Intermediate, and High Risk for Developing Postoperative Hypocalcemia After Total Thyroidectomy.
IPTH indicates intact parathyroid hormone; IV, intravenous.
SI conversion factor: To convert IPTH level to nanograms per liter, multiply by 1.0.
aPatient deemed suitable for same-day surgery by surgical team, with the absence of apparent perioperative complications and appropriate patient and/or family education and comprehension of signs and symptoms of hypocalcemia.
bIncrease in serum calcium level on 2 consecutive readings.
cDecrease in serum calcium level on a consecutive reading.
dCalcium 1200 mg in divided doses with food and vitamin D 400–800 IU daily as per recommended daily allowance.
The major limitations of this study include its retrospective nature, as well as the fact that the postoperative calcium levels recorded were not corrected for serum albumin levels, which may have falsely increased the number of patients in the mild hypocalcemia category.
Conclusions
Sex, postoperative IPTH levels, and the indication for the thyroidectomy are factors that can be used to risk stratify patients for the development of hypocalcemia after total thyroidectomy. A low postoperative IPTH level, female sex, and thyroid malignant neoplasm are all independent predictors of postoperative hypocalcemia. These variables should all be taken into account when decisions are being made about how to most effectively prevent or manage postoperative hypocalcemia after total thyroidectomy.
Acknowledgments
Funding/Support: Dr Genther is supported in part by grant T32DC000027-24 from the National Institutes of Health.
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: This study was presented at the Second World Congress on Thyroid Cancer; July 12, 2013; Toronto, Ontario, Canada.
Author Contributions: Drs Noureldine and Tufano had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Noureldine and Genther served as co–first authors, each with equal contribution to the manuscript.
Study concept and design: All authors.
Acquisition, analysis, or interpretation of data: Noureldine, Genther, Agrawal, Tufano.
Drafting of the manuscript: Noureldine, Genther, Agrawal.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Noureldine, Genther.
Administrative, technical, or material support: Agrawal, Tufano.
Study supervision: Agrawal, Tufano.
References
- 1.Lam D, Miranda R, Hom SJ. Laparoscopic cholecystectomy as an outpatient procedure. J Am Coll Surg. 1997;185(2):152–155. doi: 10.1016/s1072-7515(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 2.De Waele B, Lauwers M, Van Nieuwenhove Y, Delvaux G. Outpatient laparoscopic gastric banding: initial experience. Obes Surg. 2004;14(8):1108–1110. doi: 10.1381/0960892041975631. [DOI] [PubMed] [Google Scholar]
- 3.Thiel J, Gamelin A. Outpatient total laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 2003;10(4):481–483. doi: 10.1016/s1074-3804(05)60149-1. [DOI] [PubMed] [Google Scholar]
- 4.Sun GH, DeMonner S, Davis MM. Epidemiological and economic trends in inpatient and outpatient thyroidectomy in the United States, 1996–2006. Thyroid. 2013;23(6):727–733. doi: 10.1089/thy.2012.0218. [DOI] [PubMed] [Google Scholar]
- 5.Terris DJ, Snyder S, Carneiro-Pla D, et al. American Thyroid Association Surgical Affairs Committee Writing Task Force. American Thyroid Association statement on outpatient thyroidectomy. Thyroid. 2013;23(10):1193–1202. doi: 10.1089/thy.2013.0049. [DOI] [PubMed] [Google Scholar]
- 6.Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228 (3):320–330. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandil E, Noureldine SI, Abbas A, Tufano RP. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery. 2013;154(6):1346–1353. doi: 10.1016/j.surg.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 8.Vescan A, Witterick I, Freeman J. Parathyroid hormone as a predictor of hypocalcemia after thyroidectomy. Laryngoscope. 2005;115(12):2105–2108. doi: 10.1097/01.MLG.0000181504.69230.87. [DOI] [PubMed] [Google Scholar]
- 9.Pisaniello D, Parmeggiani D, Piatto A, et al. Which therapy to prevent post-thyroidectomy hypocalcemia? G Chir. 2005;26(10):357–361. [PubMed] [Google Scholar]
- 10.Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg. 1998;22 (7):718–724. doi: 10.1007/s002689900459. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi CP, Raffaelli M, Princi P, et al. Early prediction of postthyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 2004;136 (6):1236–1241. doi: 10.1016/j.surg.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 12.McHenry CR. “Same-day” thyroid surgery: an analysis of safety, cost savings, and outcome. Am Surg. 1997;63(7):586–590. [PubMed] [Google Scholar]
- 13.Lo Gerfo P, Gates R, Gazetas P. Outpatient and short-stay thyroid surgery. Head Neck. 1991;13(2):97–101. doi: 10.1002/hed.2880130203. [DOI] [PubMed] [Google Scholar]
- 14.Higgins KM, Mandell DL, Govindaraj S, et al. The role of intraoperative rapid parathyroid hormone monitoring for predicting thyroidectomy-related hypocalcemia. Arch Otolaryngol Head Neck Surg. 2004;130(1):63–67. doi: 10.1001/archotol.130.1.63. [DOI] [PubMed] [Google Scholar]
- 15.Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery. 2002;131(5):515–520. doi: 10.1067/msy.2002.123005. [DOI] [PubMed] [Google Scholar]
- 16.Lo CY, Luk JM, Tam SC. Applicability of intraoperative parathyroid hormone assay during thyroidectomy. Ann Surg. 2002;236(5):564–569. doi: 10.1097/00000658-200211000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payne RJ, Hier MP, Tamilia M, Mac Namara E, Young J, Black MJ. Same-day discharge after total thyroidectomy: the value of 6-hour serum parathyroid hormone and calcium levels. Head Neck. 2005;27(1):1–7. doi: 10.1002/hed.20103. [DOI] [PubMed] [Google Scholar]
- 18.Payne RJ, Tewfik MA, Hier MP, et al. Benefits resulting from 1- and 6-hour parathyroid hormone and calcium levels after thyroidectomy. Otolaryngol Head Neck Surg. 2005;133(3):386–390. doi: 10.1016/j.otohns.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Warren FM, Andersen PE, Wax MK, Cohen JI. Perioperative parathyroid hormone levels in thyroid surgery: preliminary report. Laryngoscope. 2004;114(4):689–693. doi: 10.1097/00005537-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. 2014;101(4):307–320. doi: 10.1002/bjs.9384. [DOI] [PubMed] [Google Scholar]
- 21.Demeester-Mirkine N, Hooghe L, Van Geertruyden J, De Maertelaer V. Hypocalcemia after thyroidectomy. Arch Surg. 1992;127(7):854–858. doi: 10.1001/archsurg.1992.01420070118021. [DOI] [PubMed] [Google Scholar]
- 22.Percival RC, Hargreaves AW, Kanis JA. The mechanism of hypocalcaemia following thyroidectomy. Acta Endocrinol (Copenh) 1985;109 (2):220–226. doi: 10.1530/acta.0.1090220. [DOI] [PubMed] [Google Scholar]
- 23.Kamer E, Unalp HR, Erbil Y, Akguner T, Issever H, Tarcan E. Early prediction of hypocalcemia after thyroidectomy by parathormone measurement in surgical site irrigation fluid. Int J Surg. 2009;7(5):466–471. doi: 10.1016/j.ijsu.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Erbil Y, Barbaros U, Temel B, et al. The impact of age, vitamin D3 level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am J Surg. 2009;197(4):439–446. doi: 10.1016/j.amjsurg.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Erbil Y, Bozbora A, Özbey N, et al. Predictive value of age and serum parathormone and vitamin D3 levels for postoperative hypocalcemia after total thyroidectomy for nontoxic multinodular goiter. Arch Surg. 2007;142(12):1182–1187. doi: 10.1001/archsurg.142.12.1182. [DOI] [PubMed] [Google Scholar]
- 26.Lang BH, Yih PC, Ng KK. A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg. 2012;36(6):1300–1306. doi: 10.1007/s00268-012-1561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393(5):667–673. doi: 10.1007/s00423-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita H, Noguchi S, Tahara K, et al. Postoperative tetany in patients with Graves’ disease: a risk factor analysis. Clin Endocrinol (Oxf) 1997;47(1):71–77. doi: 10.1046/j.1365-2265.1997.2201033.x. [DOI] [PubMed] [Google Scholar]
- 29.Lombardi CP, Raffaelli M, Princi P, et al. Parathyroid hormone levels 4 hours after surgery do not accurately predict post-thyroidectomy hypocalcemia. Surgery. 2006;140(6):1016–1025. doi: 10.1016/j.surg.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Scurry WC, Jr, Beus KS, Hollenbeak CS, Stack BC., Jr Perioperative parathyroid hormone assay for diagnosis and management of postthyroidectomy hypocalcemia. Laryngoscope. 2005;115(8):1362–1366. doi: 10.1097/01.MLG.0000166699.23264.37. [DOI] [PubMed] [Google Scholar]
- 31.Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery. 2003;133(2):180–185. doi: 10.1067/msy.2003.61. [DOI] [PubMed] [Google Scholar]
- 32.Bove A, Bongarzoni G, Dragani G, et al. Should female patients undergoing parathyroid-sparing total thyroidectomy receive routine prophylaxis for transient hypocalcemia? Am Surg. 2004;70(6):533–536. [PubMed] [Google Scholar]
- 33.Wilson RB, Erskine C, Crowe PJ. Hypomagnesemia and hypocalcemia after thyroidectomy: prospective study. World J Surg. 2000;24(6):722–726. doi: 10.1007/s002689910116. [DOI] [PubMed] [Google Scholar]
- 34.Erbil Y, Ozbey NC, Sari S, et al. Determinants of postoperative hypocalcemia in vitamin D-deficient Graves’ patients after total thyroidectomy. Am J Surg. 2011;201(5):685–691. doi: 10.1016/j.amjsurg.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Burge MR, Zeise TM, Johnsen MW, Conway MJ, Qualls CR. Risks of complication following thyroidectomy. J Gen Intern Med. 1998;13(1):24–31. doi: 10.1046/j.1525-1497.1998.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan I, Danila R, Aljabri H, et al. Is rapid preparation for thyroidectomy in severe Graves’ disease beneficial? the relationship between clinical and immunohistochemical aspects. Endocrine. 2008;33(2):189–195. doi: 10.1007/s12020-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 37.Zambudio AR, Rodríguez J, Riquelme J, Soria T, Canteras M, Parrilla P. Prospective study of postoperative complications after total thyroidectomy for multinodular goiters by surgeons with experience in endocrine surgery. Ann Surg. 2004;240(1):18–25. doi: 10.1097/01.sla.0000129357.58265.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McHenry CR, Speroff T, Wentworth D, Murphy T. Risk factors for postthyroidectomy hypocalcemia. Surgery. 1994;116(4):641–648. [PubMed] [Google Scholar]
- 39.Sokoll LJ, Wians FH, Jr, Remaley AT. Rapid intraoperative immunoassay of parathyroid hormone and other hormones: a new paradigm for point-of-care testing. Clin Chem. 2004;50(7):1126–1135. doi: 10.1373/clinchem.2003.030817. [DOI] [PubMed] [Google Scholar]
- 40.Richards ML, Bingener-Casey J, Pierce D, Strodel WE, Sirinek KR. Intraoperative parathyroid hormone assay: an accurate predictor of symptomatic hypocalcemia following thyroidectomy. Arch Surg. 2003;138(6):632–636. doi: 10.1001/archsurg.138.6.632. [DOI] [PubMed] [Google Scholar]
- 41.Roh JL, Park CI. Intraoperative parathyroid hormone assay for management of patients undergoing total thyroidectomy. Head Neck. 2006;28(11):990–997. doi: 10.1002/hed.20444. [DOI] [PubMed] [Google Scholar]
- 42.Flynn MB, Lyons KJ, Tarter JW, Ragsdale TL. Local complications after surgical resection for thyroid carcinoma. Am J Surg. 1994;168(5):404–407. doi: 10.1016/s0002-9610(05)80085-6. [DOI] [PubMed] [Google Scholar]
- 43.Alhefdhi A, Mazeh H, Chen H. Role of postoperative vitamin D and/or calcium routine supplementation in preventing hypocalcemia after thyroidectomy: a systematic review and meta-analysis. Oncologist. 2013;18(5):533–542. doi: 10.1634/theoncologist.2012-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 2010. [Google Scholar]
- 45.Bushinsky DA, Monk RD. Electrolyte quintet: calcium. Lancet. 1998;352(9124):306–311. doi: 10.1016/s0140-6736(97)12331-5. [DOI] [PubMed] [Google Scholar]
- 46.Nahas ZS, Farrag TY, Lin FR, Belin RM, Tufano RP. A safe and cost-effective short hospital stay protocol to identify patients at low risk for the development of significant hypocalcemia after total thyroidectomy. Laryngoscope. 2006;116(6):906–910. doi: 10.1097/01.mlg.0000217536.83395.37. [DOI] [PubMed] [Google Scholar]