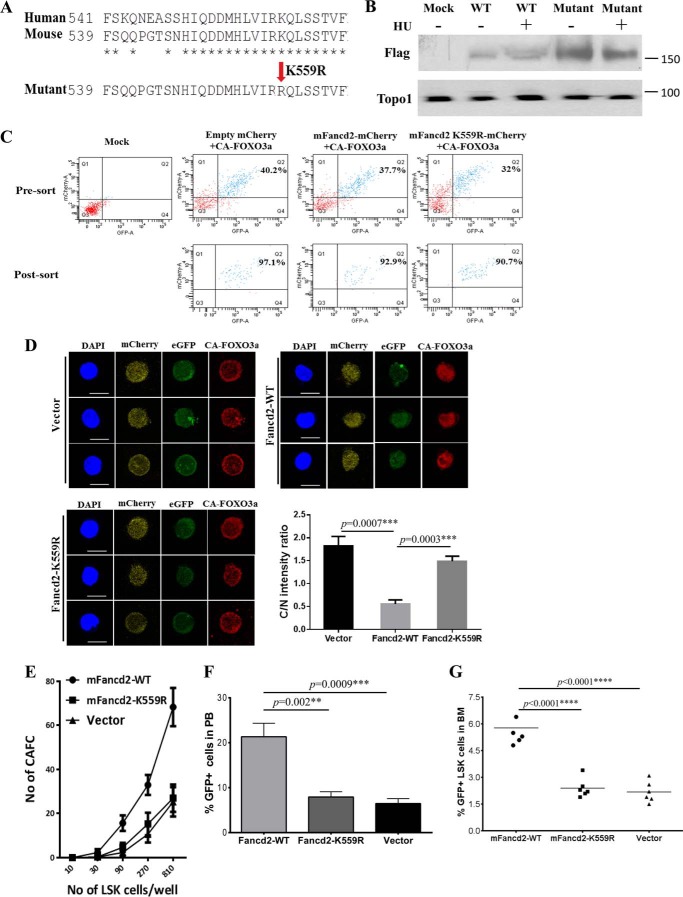
FIGURE 5.
Fancd2 and Foxo3a cooperate to maintain HSC function. A, protein sequence alignment of human and mouse Fancd2 and generation of a mutant mFancd2 that had arginine residue substituted for the lysine residue at position 559. B, WT murine embryonic fibroblast cells were transduced with lentivirus carrying empty vector, mFancd2-WT, or mFancd2-K559R and then treated with or without hydrourea (HU) for 24 h. Cell lysis were separated by SDS-PAGE gel and immunoblotted with antibodies against Flag (mFancd2) and Topo1 (loading control). C, the dKO LSK cells were co-transduced with lentivirus expressing the CA-FOXO3a-eGFP and mFancd2 WT-mCherry or mFancd2 K559R-mCherry and then sorted for double-positive (eGFP+ mcherry+) CD34−LSK cells. Shown is flow cytometry analysis of the double-positive cells before and after sorting. Mock, samples without virus. D, monoubiquitination is essential for the effect of Fancd2 on FOXO3a nuclear retention. dKO LSK cells were co-transduced with lentivirus expressing the constitutively active CA-FOXO3a with an eGFP marker (CA-FOXO3a-eGFP) (green) and the WT mouse Fancd2 with a mCherry marker (mFancd2 WT-mCherry) or its nonmonoubiquitinated mutant (mFancd2 K559R-mCherry) (yellow). Transduced CD34−LSK cells were staining by anti-FOXO3a antibody (red). Nuclei were visualized by DAPI (blue). Scale bar, 10 μm. Right panel, quantification of the fluorescence intensity of anti-Foxo3a staining in the nucleus (N) and cytoplasm (C). Each group comprises three to four mice, and 20 cells per sample. E, limiting dilution CAFC assay. dKO LSK cells were co-transduced with lentivirus expressing the WT FOXO3a with an eGFP marker and the WT mouse Fancd2 with a mCherry marker (mFancd2-WT) or its nonmonoubiquitinated mutant (mFancd2-K559R) or an empty vector. Graded numbers of double-positive (eGFP+ mcherry+) LSK cells were plated on confluent OP9 stromal cells in 96-well plates, and numbers of CAFC were counted after 4 weeks. F and G, BM transplantation assay to determine the long term hematopoietic repopulating ability. 1,000 double-positive (eGFP+ mCherry+) LSK cells (CD45.2) co-transduced with FOXO3a-eGFP and mFancd2 WT-mCherry or mFancd2 K559R-mCherry or empty-mCherry lentivirus, along with 4 × 105 recipient BM cells (CD45.1) were transplanted into lethally irradiated recipient mice. The repopulating capacity of donor HSCs was monitored by measuring percentage of GFP positive cells in the peripheral blood of the transplant recipients at 4 months post-transplantation (F). The percentage of GFP-positive LSK cells in the BM of the transplant recipients was determined at 4 months post-transplantation (G). **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.