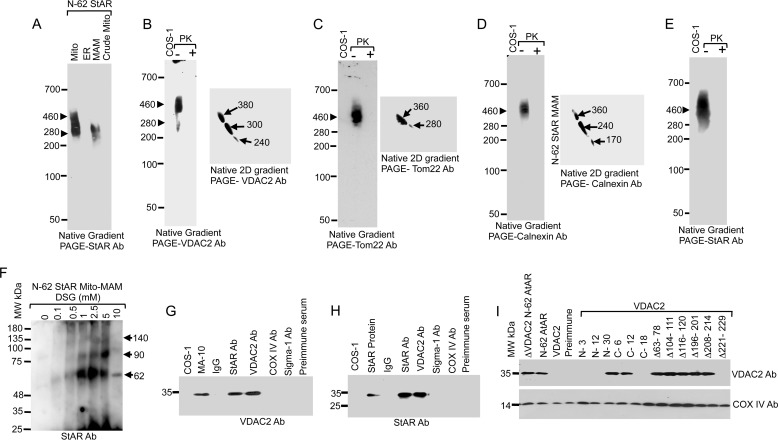
FIGURE 5.
StAR translocation in the absence of its own N-terminal sequence. A, analysis of cellular fractions by native gradient PAGE in COS-1 cells overexpressing N-62 StAR and probed with the StAR antibody (Ab). B–D, identification of the MAM-associated proteins of COS-1 cells that overexpress N-62 StAR. The MAM fractions were analyzed by one-dimensional native gradient PAGE followed by two-dimensional (2D) analysis of the excised band from the one-dimensional native PAGE. The identity of the complexes was visualized after staining with the indicated antibodies. E, analysis of the MAM fractions by native gradient PAGE with and without incubation with proteinase K (PK) and staining with a StAR antibody. F, the MAM fraction of N-62 StAR-expressing cells was cross-linked with increasing concentrations of the chemical cross-linker DSG, separated by SDS-PAGE, and stained with a StAR antibody. Three cross-linked products of 62, 90, and 140 kDa band were identified. The MAM fraction was immunoprecipitated with the indicated antibodies and blotted with VDAC2 (G) and StAR (H) antibodies. I, ΔVDAC2 COS-1 cells overexpressing N-62 StAR were transfected with the indicated VDAC2 deletion mutants, immunoprecipitated with a StAR antibody, and immunoblotted with a VDAC2 antibody. Deletion of more than 18 amino acids from the C terminus ablated VDAC2 interaction with StAR, and similarly the region of amino acids 221–229 was equally important, but deletion of any amino acids from the first N-terminal sequence ablated interaction with StAR. The bottom panels probed with COX IV antibody show as equivalent amount of protein present in each lane. Mito, mitochondria; Mito-MAM, crude mitochondria.