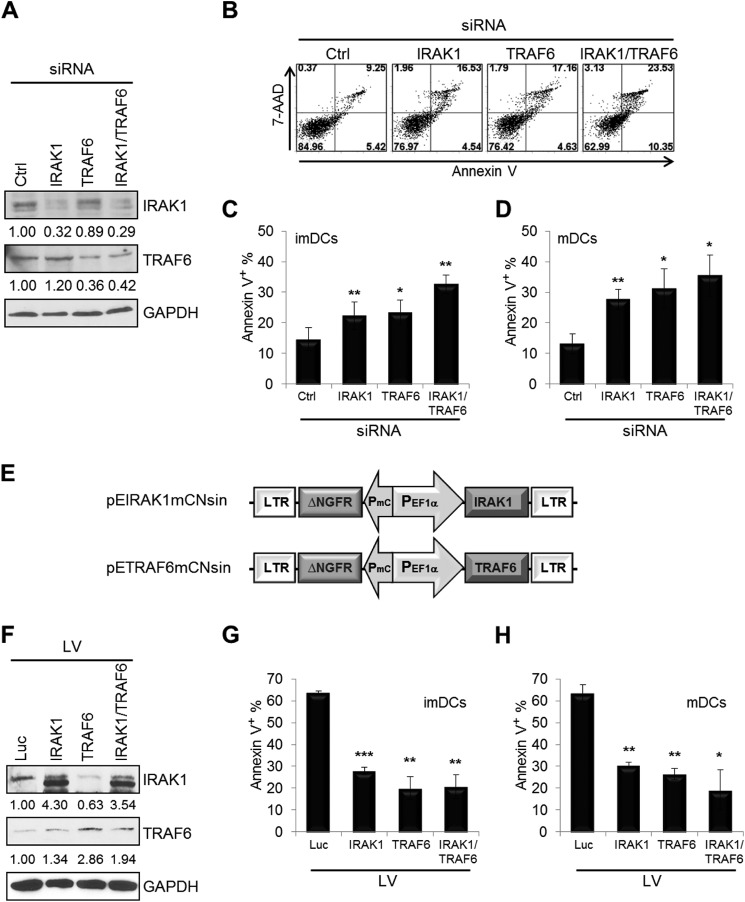
FIGURE 4.
TRAF6 and IRAK1 modulate DC apoptosis. imDCs at day 4 were transfected with TRAF6 and/or IRAK1 siRNA and scrambled control (Ctrl) siRNA at a final concentration of 30 nm for 40 h. A, reduced expression of TRAF6 and/or IRAK1 was analyzed by Western blotting. B, all cells were analyzed for apoptosis by staining with FITC-annexin V and 7-aminoactinomycin D (7-AAD). C, the percentages of annexin V+ cells (including annexin V+/propidium iodide− and annexin V+/propidium iodide+) were determined as in B. D, imDCs at day 6 were transfected with TRAF6 and/or IRAK1 siRNA and scrambled control siRNA, followed by the maturation mixture for 40 h, and cells were then analyzed for apoptosis as described above. E, schematic representation of the pEhfflucmCNsin lentiviral vector expressing human IRAK1 and TRAF6. LTR, long terminal repeat; mC, minimal core promoter element; EF1α, elongation factor 1α. imDCs at days 4 (G) and 6 (F and H) were transduced with the control lentiviral vector (Luc) and with the lentiviral vector (LV) expressing TRAF6 and/or IRAK1, and cells were then incubated for 48 h with (F and H) or without (G) the maturation mixture. The transduction efficiency was determined to be >80% NGFR+ by flow cytometry. Cells were analyzed for Western blotting (F) or apoptosis (G and H) by staining with FITC-annexin V and allophycocyanin-NGFR. NGFR+ cells were used only to determine the percentages of annexin V+ cells. High baseline apoptosis in the control lentiviral vector-transduced imDCs and mDCs was due to use of Polybrene for lentiviral transduction and serum-free AIM V medium for DC culture. Data shown are mean percentages ± S.E. and are representative of four (A–D) or two (F–H) independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005 (by paired t test).