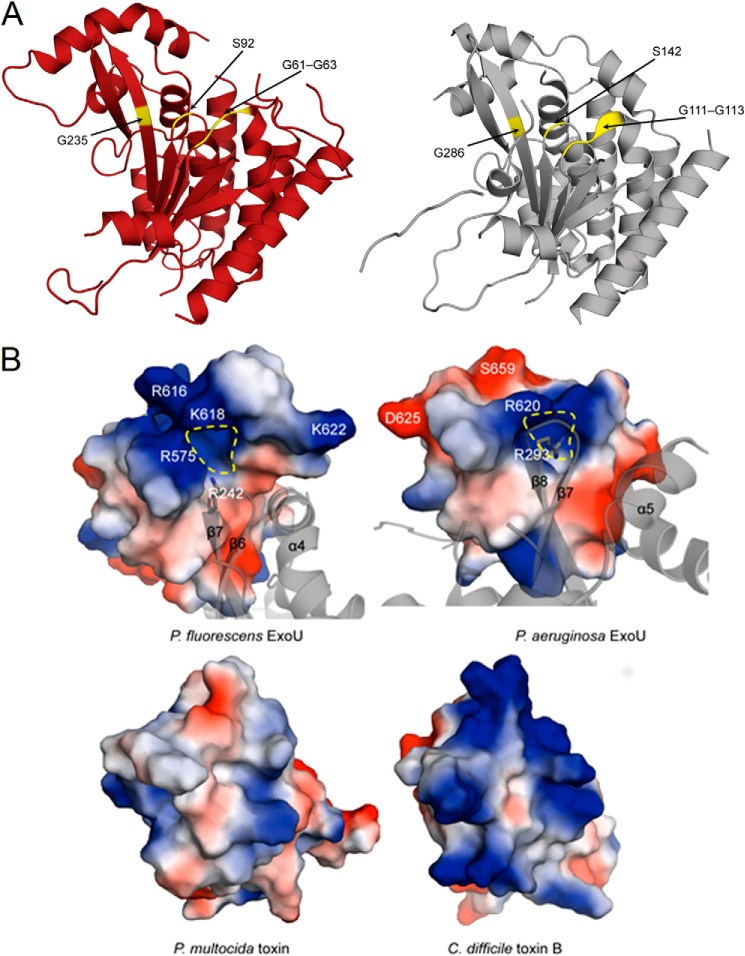
FIGURE 10.
Comparisons of the catalytic regions and surface charge distributions of ExoU proteins. A, comparison of the catalytic patatin PLA2 domain of ExoUP. flu (red, chain A used) and ExoUP. aer (silver). The glycine-rich oxyanion hole region (Gly-61–63 in ExoUP. flu and Gly-111–113 in ExoUP. aer), catalytic serine residue (Ser-92 in ExoUP. flu and Ser-142 in ExoUP. aer), and an additional conserved glycine residue important in activity (Gly-235 in ExoUP. flu and Gly-286 in ExoUP. aer) are highlighted. B, charged surface and ribbon representations of the domain four portions of ExoUP. flu and ExoUP. aer and the corresponding portions of the PMT and C. difficile toxin B (TcdB). Dashed yellow line indicates a potential PI(4,5)P2-binding/recognition site.