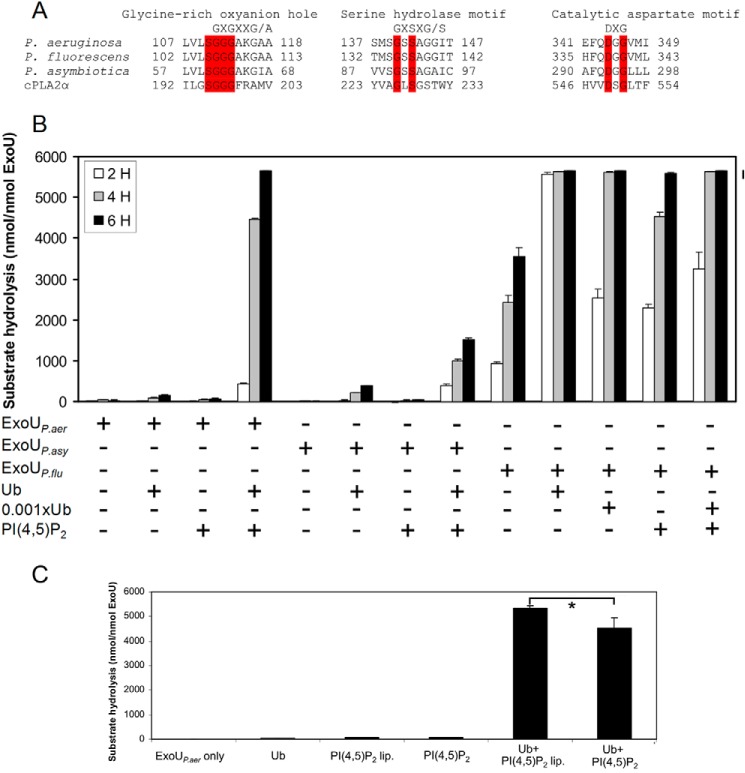
FIGURE 6.
ExoUP. flu and ExoUP. asy have PLA2 activity. A, ExoUP. aer and its homologs from P. fluorescens and P. asymbiotica were aligned with cPLA2 (accession number P47712.2) at their putative catalytic sites. Residues highlighted in red are identical in each of these proteins. B, PLA2 activity of each of these proteins was measured in vitro using a synthetic phospholipid substrate. Each protein was analyzed for its ability to hydrolyze substrate by itself, with ubiquitin, with PI(4,5)P2, or with both ubiquitin and PI(4,5)P2. Ubiquitin (Ub) was added at a 1:1 ubiquitin/ExoU molar ratio or at a 1:1000 ratio (0.001×Ub). C, PLA2 activity of ExoUP. aer was measured with the addition of ubiquitin, PI(4,5)P2, or PI(4,5)P2-containing liposomes with the same overall PI(4,5)P2 concentration (PI(4,5)P2 lip.). PLA2 activity was measured at 5 h. Each assay was performed in triplicate; values are means, and error bars are S.E. *, p < 0.01.