FIGURE 1.
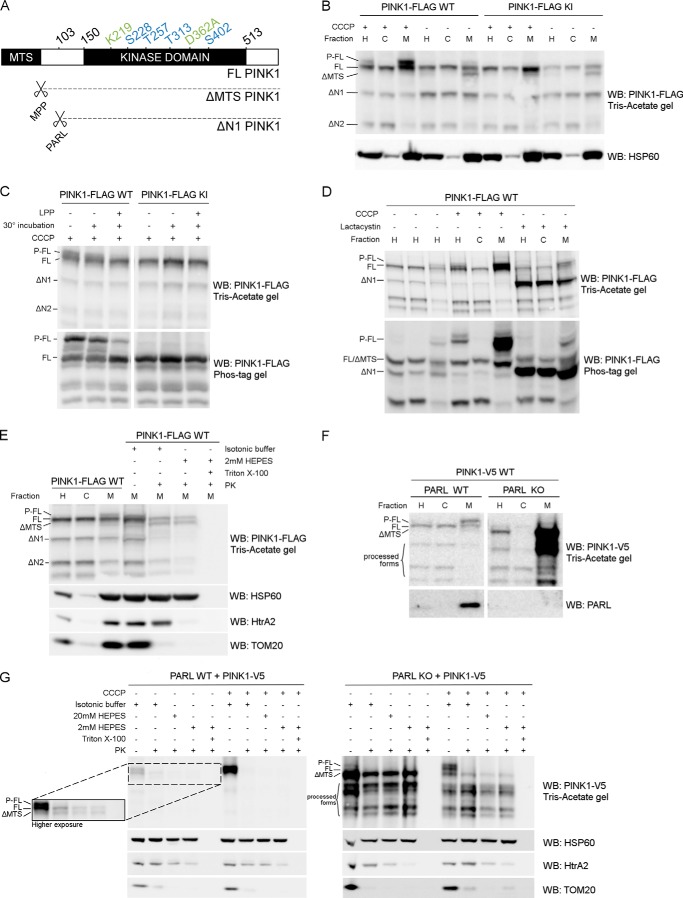
Phosphorylated PINK1 is present at the outer mitochondrial membrane. A, schematic representing FL PINK1, including the kinase domain and MTS. MPP cleaves off the MTS, resulting in ΔMTS PINK1, and further processing by PARL at residue 103 results in the ΔN1 PINK1 product. The four phosphorylation sites analyzed in this study (blue) and the sites mutated in KI (green) PINK1 are indicated. B, HEK cells transiently transfected with WT or KI FLAG-tagged human PINK1 and treated with 10 μm CCCP for 3 h were fractionated in postnuclear or homogenate fraction (H), and subsequent H fraction was further centrifuged at 7000 × g to obtain the mitochondrially depleted or cytosol-enriched (C) and mitochondrially enriched (M) fractions, respectively. Expression of PINK1 was evaluated after SDS-PAGE on 7.5% Tris acetate gel and immunoblotted using anti-FLAGM2. Immunoblotting for the mitochondrial marker HSP60 served as a fractionation quality control. For WT, but not KI, a doublet was observed that corresponded to the phosphorylated and non-phosphorylated FL form of PINK1 (P-FL and FL, respectively). Further processed PINK1 forms are indicated as ΔMTS, ΔN1, and ΔN2. WB, Western blot. C, mitochondrially enriched fractions, obtained from HEK cells transfected with WT and KI PINK1-FLAG and incubated with 10 μm CCCP for 3 h, were treated with LPP at 30 °C. Samples were analyzed by SDS-PAGE on a 7.5% Tris acetate gel and 7.5% Phos-Tag gel and immunoblotted with anti-FLAGM2 antibody for PINK1 detection. A control for loss of phosphorylation by 30 °C incubation alone was included. Phosphatase treatment confirmed that the upper band (P-FL) was a phosphorylated form of PINK1. D, fractionation of HEK cells treated for 3 h with either 10 μm CCCP or 10 μm lactacystin was analyzed by SDS-PAGE on a 7.5% Tris acetate or 7.5% Phos-Tag gel and further immunoblotted for PINK1 detection using anti-FLAGM2 antibody. Mitochondrial PINK1 was phosphorylated under control (DMSO) conditions but accumulated upon CCCP-induced depolarization. Proteasomal blockage with lactacystin also led to a modest increase of P-FL PINK1 in addition to accumulation of the ΔN1 processed form. E, mitochondrially enriched fractions of MEF cells stably expressing FL PINK1-FLAG were subjected to a PK sensitivity assay to assess submitochondrial localization of P-FL PINK1. P-FL is PK-sensitive, whereas non-phosphorylated FL PINK1 and PINK1 lacking the mitochondrial targeting sequence (ΔMTS) are at least partially protected from PK even under hypotonic conditions (2 mm HEPES). As a control for protein digestion under each condition, fractions were also immunoblotted using antibodies against the outer membrane protein TOM20, intermembrane space protein HtrA2, and matrix protein HSP60. F, MEFs derived from PARL WT and PARL KO mice stably expressing WT PINK1-V5 were fractionated and expression of PINK1 was evaluated after SDS-PAGE and anti-V5 immunoblotting. ΔMTS PINK1 accumulates in PARL KO cells and further N-terminal processing is altered leading to an accumulation of PINK1 C-terminal fragments in the mitochondria (M) of PARL KO cells. G, mitochondrially enriched fractions from PARL WT and KO MEFs stably expressing WT PINK1-V5 were subjected to PK treatment under isotonic and hypotonic (20 mm and 2 mm HEPES) conditions in the presence or absence of detergent. Equal amounts were loaded for PARL WT and KO. A higher exposure inset for PARL WT allows better analysis of PINK1 expression under these conditions. ΔMTS PINK1 was cleaved by PARL and accumulated in PARL KO mitochondria but was only digested by PK in the presence of detergent. Depolarization induced by CCCP led to the accumulation of P-FL PINK1, sensitive to proteinase K under isotonic conditions. Therefore, the accumulation caused by the absence of PARL or by CCCP-induced depolarization concerns different PINK1 forms present in different submitochondrial compartments. As a control, the PK sensitivity of the outer membrane protein TOM20, intermembrane space protein HtrA2, and matrix protein HSP60 was evaluated by immunoblotting.