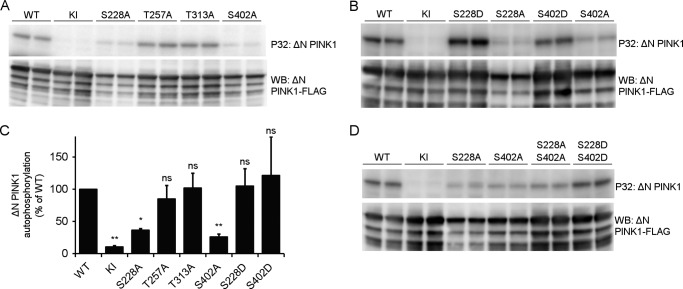
FIGURE 5.
Autophosphorylation of ΔN PINK1 occurs at residues Ser-228 and Ser-402. A, an in vitro phosphorylation assay using [γ-32P]ATP was performed with purified human ΔN PINK1 harboring phosphodead mutations on four putative phosphosites. The phosphomutants S228A and S402A showed reduced phosphorylation, whereas mutation of Thr-257 and Thr-313 did not affect ΔN PINK1 autophosphorylation when compared with WT. B, in vitro phosphorylation assay using [γ-32P]ATP and purified ΔN PINK1 with phosphomimetic and phosphodead mutant PINK1 for residues Ser-228 and Ser-402 shows that a phosphomimetic mutant PINK1 can restore PINK1 phosphorylation levels observed for the corresponding phosphodead mutation. C, quantification of ΔN PINK1 autophosphorylation relative to WT (mean ± S.E., n = 3 independent experiments). Statistical significance was calculated between each mutant and WT ΔN PINK1 using Dunnett's test. *, p < 0.05; **, p < 0.01; ns, nonspecific. D, in vitro phosphorylation assay using [γ-32P]ATP and purified ΔN PINK1 with a combined S228A and S402A mutation shows decreased ΔN PINK1 autophosphorylation levels comparable with the levels for the single mutations, indicating the existence of residual phosphorylated residue(s) on PINK1. The combined phosphomimetic mutation S228D and S402D shows increased in vitro autophosphorylation levels, showing that phosphorylation of these two residues increases PINK1 kinase activity and phosphorylation of the residual phosphoresidue(s). Immunoblot analysis using anti-FLAGM2 shows that equal amounts of PINK1 were applied.