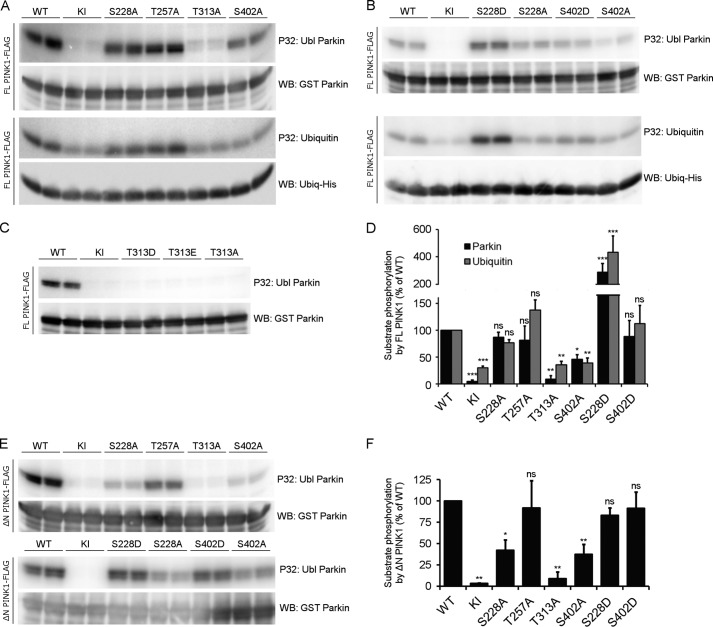
FIGURE 6.
The residues Thr-313 and Ser-402 are important for substrate phosphorylation. A, an in vitro phosphorylation assay using [γ-32P]ATP was performed on purified FL PINK1 with purified Ubl Parkin or His-tagged Ubiquitin. Autoradiographic exposure shows that, for FL PINK1, mutation of Ser-228 and Thr-257 does not affect Parkin or Ubiquitin phosphorylation. Mutation of Thr-313 completely abrogates substrate phosphorylation, whereas the S402A mutation leads to a substantial decrease. There is still phosphorylation detectable for KI PINK1, indicating the presence of a contaminating kinase capable of phosphorylating Ubiquitin at low levels. Immunoblot analysis using anti-GST or anti-His confirms that equal amounts of Parkin and Ubiquitin were applied. WB, Western blot. B, an in vitro phosphorylation assay using [γ-32P]ATP, FL PINK1, and Ubl Parkin or Ubiquitin shows that the S228D and S402D mutations increase substrate phosphorylation. The S228D mutation increases substrate phosphorylation beyond WT levels, which are comparable with that obtained for S228A PINK1. The decreased Parkin phosphorylation for FL S402A PINK1 can be rescued by a phosphomimetic mutation, S402D. C, an in vitro phosphorylation assay using [γ-32P]ATP was performed on purified FL PINK1 mutated at the Thr-313 residue with purified Ubl Parkin. Phosphomimetic (T313D or T313E) mutation of Thr-313 does not rescue the decrease in Parkin phosphorylation observed for T313A. D, quantification of Ubl Parkin and Ubiquitin phosphorylation relative to WT. Statistical significance was calculated between each mutant and WT FL PINK1 using Dunnett's test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, nonspecific. Data are mean ± S.E. (n = 3 independent experiments). E, an in vitro phosphorylation assay using [γ-32P]ATP was performed using purified ΔN PINK1 and purified Ubl Parkin. Similar to FL PINK1, Parkin phosphorylation is abolished upon T313A mutation in ΔN PINK1, whereas the S228A and S402A mutations reduce it significantly. The S228D and S402D phosphomimetic mutations restore Parkin phosphorylation back to WT levels. F, quantification of Ubl Parkin phosphorylation by ΔN PINK1. Statistical significance was calculated between each mutant and WT ΔN PINK1 using Dunnett's test. *, p < 0.05; **, p <0.01; ns, nonspecific. Data are mean ± S.E. (n = 3 independent experiments).