FIGURE 8.
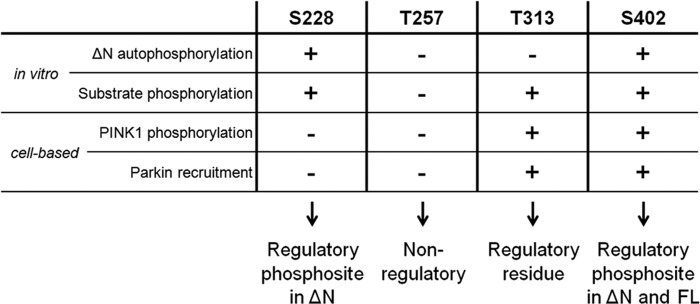
Overview of the role of Ser-228, Thr-257, Thr-313, and Ser-402 residues in PINK1. Ser-228 is an autophosphorylation site in ΔN PINK1, and it can regulate substrate phosphorylation in vitro. However, both in vitro and in cells, Ser-228 phosphorylation plays no role in the regulation of WT FL PINK1 activity. Nevertheless, we propose it as a regulatory phosphosite for processed PINK1. We found no implication for Thr-257 as a (regulatory) phosphosite in any of our experimental setups and, therefore, propose that this putative phosphosite has no functional role for PINK1 activity. Although Thr-313 is an essential residue for PINK1, its function is not regulated through phosphorylation because autophosphorylation is not affected upon Thr-313 mutation, and phosphomimetics rescue none of the observed functional defects. Like Ser-228, Ser-402 is an autophosphorylation site in ΔN PINK1, but it also regulates FL PINK1 in vitro and in cells. We propose this residue as a regulatory phosphosite for both FL and processed PINK1.
