Introduction
The “New discipline for blood transfusion activities and national production of blood derivatives” (Italian Parliament, October 21st, 2005) brought about a significant update of all regulatory aspects concerning transfusion medicine activities in Italy, including the establishment of a nationally co-ordinated blood system consistent with the autonomy of regional authorities1. The aim was to identify homogeneous standards of quality and safety nationwide1. In this setting, the newly born Italian National Blood Centre was to be responsible for all technical and scientific aspects related to transfusion medicine issues, including (i) blood and blood product self-sufficiency; (ii) blood quality and safety; (iii) appropriate utilisation of blood resources; (iv) accreditation and funding of transfusion medicine activities; (v) a national blood information system; (vi) technology assessment; (vii) external quality assessment; (viii) a national haemovigilance network; (ix) control of medical products deriving from human plasma; (x) inspections and controls of the plasma-derivative industry; (xi) education and scientific research in transfusion medicine, and (xii) promotion of voluntary, non-remunerated, responsible and periodic blood donation1.
In the light of such an ambitious agenda, the National Blood Centre started an extensive series of scientific initiatives, including an extensive collaboration with the laboratory of proteomics and metabolomics at Tuscia University, Viterbo, Italy. The present review summarises the main outcomes of this joint effort, while putting the results in the broader framework of laboratory research in the field of transfusion medicine, with a special focus on the storage of red blood cell (RBC) concentrates.
“-Omics” and transfusion medicine: first contacts and rendezvous
Since its establishment, the National Blood Centre was eager to contribute to a deeper understanding of the molecular mechanisms underpinning the safety and efficacy of blood-derived therapeutics. From a perspective of laboratory science, this involved contact with research institutions with expertise in one of the most rapidly emerging analytical technologies of the last decade, proteomics. Indeed, during the last 10 years, the scientific community has witnessed an exponential diffusion of laboratory investigations in the field of “-omics” sciences in transfusion medicine2,3.
“-Omics” sciences can be defined as analytical disciplines in which the bulk of research is focused on the qualitative and quantitative measurement of all the genes (genomics), mRNA transcripts (transcriptomics), proteins (proteomics) or metabolites (metabolomics) in a given sample2. Early systematic reviews in the field convinced the National Blood Centre that this was a path to explore, an intuition that led to the creation and expansion of a network of connections with several groups involved in this research endeavour. During the last decade, National Blood Centre institutions across Europe began to approach university and clinical laboratories, but the rendezvous between these formerly distant worlds was reached and consolidated through the promotion of editorial initiatives (the interested reader is referred to the special issues of Blood Transfusion4,5 and the Journal of Proteomics6,7).
The implementation of “-omics” in the field of transfusion medicine enabled determination of molecular parameters affecting storage quality of platelet concentrates8,9, and the effect of different pathogen inactivation technologies on the proteomic and metabolomic profiles of platelets in the blood bank10,11. “-Omics” can also be exploited to perform heterogeneity evaluation assessments of plasma-derived or recombinant coagulation factors, such as factor VIII12,13. Notably, the most valuable insights gained through “omics” research in the field of transfusion medicine are related to the storage of erythrocyte concentrates, as discussed extensively in this review.
Issues related to the duration of red blood cell storage
Despite almost a century of improvements in the field of RBC storage, in 2008 controversial retrospective studies were published in a prestigious journal14; in brief, the authors highlighted a statistically significant, increased likelihood of morbidity and mortality in patients undergoing cardiac surgery when transfused with units stored for more than 14 days. Although the statistical validity of the results was immediately questioned and controversial data were obtained through extensive retrospective investigations of the literature15, the bulk of attention concerning RBC storage became focused on three main questions: (i) how long is too long?16 (ii) is RBC storage duration the critical issue, or should we be seeking to improve storage quality?17 (iii) to what extent could improvements be effective enough to guarantee a safe and effective blood-derived therapeutic product?18
Although randomised, prospective clinical trials could probably provide some tentative answers to these questions19, any efforts made to improve standards of storage quality should start from the achievement of a thorough understanding of storage quality from clinical and biomedical standpoints. Most of the reported early results of the prospective, clinical trials currently underway (ABLE, ARIPI, RECESS, TRANSFUSE- reviewed by Grazzini et al.19) are conflicting, with some confuting and others confirming a correlation between storage duration and an increased likelihood of adverse events in certain categories of recipients (e.g. critically ill, traumatised, peri-operative patients).
Conversely, accumulating biomedical evidence (including results from laboratories performing proteomic and metabolomic investigations on RBC storage) suggests that storage duration might be related to a progressive accumulation of detrimental changes to RBC physiology and function (the so-called “storage lesions”)20–23. While the clinical relevance of the storage-dependent accumulation of lesions is still matter of debate, the overlap between the temporal sequence of such events (mostly accumulating in a statistically significant fashion after the second week of storage) and Koch’s retrospective observations16 appears to be more than coincidental.
“-Omics” and red blood cells
RBC have always been deemed one of the easiest cell models in biology. Indeed, mature erythrocytes are devoid of organelles and nuclei, which impairs their capacity for de novo protein synthesis and would theoretically promote proteome stability. However, it is worth noting that haemoglobin alone accounts for 98% of the cytosolic proteome and 92% of the cell’s dry weight. Therefore, any attempt to delve into the biological complexity of these cells should include a reduction of the analytical “noise” constituted by the overwhelming abundance of haemoglobin, in order to be able to focus on the residual components of the protein apparatus. In this view, several pre-fractionation strategies have been introduced over the years, involving either the adoption of antibodies to haemoglobin for its targeted removal or the application of native gel-based preparative approaches24. Among these pre-fractionation strategies, the use of combinatorial hexapeptide ligand libraries has enabled the detection of 1578 unique gene products in the cytoplasm of RBC, a finding that has paved the way for bioinformatic processing of protein-protein interactions, revealing the interconnections between energy and redox metabolism in mature erythrocytes25. These in silico predictions have recently been confirmed by the results of native gel-based approaches, with the identification of at least 55 multi-protein complexes in the cytosol of mature erythrocytes26. Drawing on these findings, Goodman and colleagues recently put the pieces together, compiling the most extensive list of RBC cytosolic and membrane proteins to date, a list formed of 2,289 entries27. This is relevant in that, before focusing on the understanding of the events targeting RBC during prolonged storage in a blood bank, it is essential to understand RBC biology, which in turn affects erythrocyte ageing in vivo (senescence)28 and the responses of these cells to the artificial preservation conditions (hypothermia, plastic bags, additives)23 they are subjected to during hypothermic liquid storage (in vitro ageing). Finally, translation of the findings of these biomedical investigations into transfusion medicine-relevant insights requires further understanding of the effects that “storage lesions” might have when the RBC are transfused into a recipient. Although there would be serious ethical concerns about conducting human studies with this scope, it is worth noting that recently conceived in vitro and animal models (murine, ovine and canine models)29–31 could speed up determination of the biomedical implications related to the transfusion of “older” units from the perspective of laboratory science.
Despite all the advances summarised above, large-scale proteomic investigations on blood donated by healthy human volunteers is still a challenging task, since donor variability32 is a critical issue that basically hampers the generalisation of any laboratory results and knowledge transfer into transfusion medicine practices.
Storage-dependent alterations in red blood cell oxygen affinity and ion homeostasis
Prolonged storage of RBC results in increased oxygen affinity after 14 days of storage, although haemoglobin interactions with oxygen are apparently preserved throughout the whole shelf-life of packed red cells33,34. One of the leading causes underpinning this phenomenon is the progressive consumption of the high-energy phosphate compound 2,3-diphosphoglycerate (2,3-DPG). Since DPG stabilises the tense “T” (deoxygenated) state of haemoglobin, its concentration is inversely proportional to oxygen affinity. On the other hand, acidification of the internal and external pH during prolonged storage, as a result of glycolytic metabolism, promotes oxygen off-loading through the so-called Bohr effect22.
Other than pH, prolonged storage of RBC also results in the deregulation of cation homeostasis, with the progressive leakage of potassium in the supernatant and intracellular accumulation of calcium35,36. Hyperkalaemia might result in complications in certain categories of recipients, such as paediatric patients. Accumulation of intracellular calcium is associated with deregulation of energy and redox metabolism (consumption of high-energy phosphate compounds, such as adenosine triphosphate [ATP])35, which fuels the activity of calcium pumps.
Storage duration and metabolic deregulation
Storage at 4 °C in the blood bank results in decreased activity of most of the enzymes involved in energy metabolism. This leads to a progressive reduction of the high energy-phosphate compounds ATP and DPG35 which, even if rapidly restored upon transfusion10, would impair RBC survival in the bloodstream of the recipient.
Simultaneously, progressive accumulation of the glycolytic by-product lactate in the supernatant of packed red cells is a common feature during prolonged storage, independently of the additive solutions used35–41.
Notably, during the first 2 weeks of storage there is a build up of early glycolytic intermediates and progressive accumulation of pentose phosphate pathway (PPP) metabolites36,38,39. This is relevant in that the PPP fuels the generation of Reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH), which in turn promotes anti-oxidant defences by taking part in the conversion of oxidized glutathione back to its reduced form. However, as storage continues, RBC appear to lose the capacity to build up anti-oxidant potential via the PPP36,37. Indeed, accumulation of reactive oxygen species (ROS) seems to be irreversible from the second week onwards, when ROS levels reach their peak35. Of note, blood donated by glucose 6-phosphate dehydrogenase (G6PDH)-deficient donors should raise additional concerns in this sense, in that G6PDH is the rate-limiting enzyme of the PPP and thus its natural absence might result in faster accumulation of oxidative stress in units donated by subjects with this enzyme deficiency42.
The progressive deregulation of glutathione homeostasis during storage of RBC in the blood bank is now a consolidated finding43. However, only with the introduction of “-omics” technologies (in particular, metabolomics) was it possible to observe a progressive deregulation of amino acid metabolism, especially of the glutathione precursors glutamate and cysteine, which is proportional to storage duration36,39.
ROS accumulation is a biological constant in RBC, considering that the main purpose of these cells is to transport oxygen and the core of the protein-protein interaction network is devoted primarily to the maintenance of anti-oxidant defences25,27. In this view, it is worth recalling that the challenge of haemoglobin oxidation is the main burden in RBC physiology, since the presence of oxygen and haem iron results in the promotion of redox reactions (Haber-Weiss and Fenton) ultimately leading to the accumulation of ROS. Significant advances have been made in this regard though the introduction of leucofiltration strategies, since white blood cell removal reduces the accumulation of ROS.35
Accumulation of oxidative stress results in protein fragmentation and impairment of the band 3 transport metabolon
Exacerbation of oxidative stress as storage progresses results in ROS targeting the protein and lipid fractions44.
Proteins targeted by ROS are either carbonylated35,45, fragmented or aggregated46–48 or relocated to the membrane35,49 and thereby vesiculated (as discussed below). Oligomerisation state and thus functional activity of key antioxidant enzymes such as peroxiredoxin 2 is also influenced by storage duration49.
The anion exchanger 1 protein band 3 is among the key targets of protein fragmentation/aggregation events during prolonged storage35,50. In this view, it is worth noting that fragmentation of the N-terminal cytosolic domain of band 3 is promoted by both ROS and enzymatic cleavage50, through a process that promotes suicidal death of erythrocytes, erypotsis, in an apoptotic-like fashion, which is to be distinguished from normal senescence occurring in vivo51.
Band 3 protein, the most abundant erythrocyte membrane protein with million of copies per cell, plays a central role in erythrocyte physiology, because of its activity in the “transport metabolon”52. Indeed, band 3 is involved in the modulation of oxygen transport by influencing the intracellular pH (and thus oxygen off-loading) through its regulation of the chloride shift (exchange of Cl−/HCO3− anions). Furthermore, the N-terminal cytosolic domain of band 3 is an inhibitory docking site for glycolytic enzymes, although such inhibitory binding is challenged by deoxyhaemoglobin translocation to the negatively charged N-ter tail of band 3, which delocalises and thus reactivates glycolytic enzymes in an oxygen-dependent fashion52.
Oxidation to proteins does not only result in increased carbonylation, but also in the progressive accumulation of advanced glycation end-products (non-enzymatic addition of sugar moieties to the proteins) in the membrane53 and cytosol54, where glycated forms of haemoglobin (Hb1Ac) accumulate proportionally to storage duration. Notably, alterations to Hb1Ac levels in units that have been stored for longer periods might cause problems when treating/monitoring certain categories of patients, such as those suffering from diabetes. In parallel, altered membrane protein glycosylation patterns might influence the rheological properties of RBC55.
Another oxidative stress-dependent parameter influenced by continued storage is protein nitrosylation, including nitrosylation of cystein β93, which influences nitric oxide metabolism and vasodilatory effects mediated by erythrocytes upon transfusion34. Further information will soon become available from the application of proteomic technologies to post-translational modifications of RBC in the blood bank, especially in the light of the emerging role of endothelial nitric oxide synthase and arginase in the modulation of nitric oxide metabolism in mature erythrocytes56.
Morphological alterations and microvesicles
ROS targeting the lipid fraction result in the accumulation of pro-inflammatory mediators, such as prostaglandins (for example, 8-isoprostane36). However, prolonged storage also results in the alteration of the lipid composition of the membrane, with the preferential loss of certain classes of lipids and enrichment of others in the membrane fraction, such as glycerophosphoserines and ceramides57,58.
Together with the above-mentioned alterations to structural proteins (especially cytoskeletal proteins), these changes promote reorganisation of the membrane59 and exacerbate the naturally occurring phenomenon of membrane loss known as vesiculation. In an apoptosis-like fashion, stored erythrocytes continuously shed sub-micron vesicles released from membrane blebs, a process that is accompanied by the progressive loss of the normal discocytic phenotype and the acquisition of an echinocytic or sphero-echinocytic shape, to a significant extent from the second week of storage onwards35,59.
Alterations to the lipid fraction and membrane composition also end up influencing RBC deformability59,60, and consistently affect the capacity of the cells to cope with osmotic stress59,61. These parameters are also affected by progressive leaching of the plasticizer di(2-ethylhexyl)phthalate from plastic bags and its intercalation into cell membranes. Although di(2-ethylhexyl)phthalate has been reported to halve haemolysis by the end of the storage period and promote RBC survival, it does so at the expense of cell deformability62 and is a potentially toxic compound. In the light of this, RBC storage containers formulated with alternative plasticizers are currently under evaluation62.
Overall, lipid raft rearrangement, membrane blebbing, vesiculation, caspase activation and phosphatidylserine exposure are all features shared by RBC ageing in the blood bank and those undergoing apoptosis (eryptosis)63.
Over the years, proteomic approaches have been used to document the protein composition of RBC supernatants64,65 and RBC-shed microvesicles66–69. Notably, vesicles expose phosphatidylserine and preserve the rheological properties of donor erythrocyte membranes67, which would result in pro-immunogenic potential through the promotion of erythrocyte phagocytosis in the bloodstream of the recipient. In addition, they seem to be enriched in oxidized proteins69, as if vesiculation were a self-protective mechanism to ensure removal of completely compromised proteins70.
Alterations to membrane proteins and morphology, formation of band 3 oligomers and phosphatidylserine exposure end up compromising the survival of transfused, long-stored erythrocytes, since as many as 25% of the transfused RBC do not survive more than 24 hours upon transfusion into the bloodstream of a recipient71,72.
Finally, proteomic technologies have helped to identify potential markers of storage quality, such as the anti-oxidant protein peroxiredoxin 2, which migrates to the membrane in proportion to the duration of storage73. Alternative markers of ageing have been proposed that could function as indirect indicators of the oxidative state of erythrocytes in a unit; such a marker has been incorporated in recently designed, inkjet-printed carbon nanotubes for antioxidant assays in blood bags74.
Another, unpredicted application of these markers might be the detection of autologous blood doping in endurance sport athletes75, a practice for which no judicially accepted strategy for doping detection exists, except for the biological passport (a long-term record of an athlete’s haematocrit, reticulocyte count and haemoglobin concentration)76.
Alternative storage strategies
In the light of the controversial clinical results but apparently converging laboratory evidence about the reduced safety and effectiveness of erythrocyte concentrates stored for more than 2 weeks, one could wonder why National Blood Centres do not promote theoretically safer and more restrictive standards regarding the shelf-life of packed red cells. The answer to this question is both immediate and rational: shortening the shelf-life of RBC in the absence of conclusive evidence would result in potential blood product shortages, as confirmed by bioinformatic predictive simulations77, with no guaranteed improvements for the recipients. However, while clinical trials may ultimately conclude that shortening the shelf-life of erythrocyte concentrates would have significant advantages for recipients, National Blood Centres will probably seek alternative products or storage strategies to complement current practices in order to meet their national blood demands. Indeed, alternative storage strategies have long been under evaluation, and proteomics and metabolomics have contributed useful insights in this field as well.
Cryostorage
Proposed decades ago, cryopreservation of packed red cells is one of the earliest alternative storage strategies. Encouraging results have been reported over the years, indicating that erythrocytes stored at temperatures as low as −80 °C in the presence of low doses of cryoprotectants, such as 40% glycerol (there are also high glycerol variants, with different rapidity of freezing time and temperature), can remain viable for as long as 30 years78. Deglycerolisation, washing and resuspension in additive solution-1 (AS-1) or -3 (AS-3) has been reported to promote survival of thawed erythroyctes for a further 3 weeks78.
Recently, metabolomics and scanning electron microscopy analyses of cryopreserved erythrocytes were performed to monitor cell processing steps (from fresh blood, to glycerolisation, thawing and deglycerolisation/washing)79. Cell processing for cryostorage resulted in increased RBC volumes and shape alterations, leading to increased osmotic fragility and permeability to ions79. A significant drop in pH was observed which could not to be attributed to a higher metabolic rate, since the intracellular levels of lactate did not show substantial fluctuation in this study79. Membrane anomalies were thought to be related to the observed haemolysis, which preferentially affected the densest and oldest cell sub-populations, as confirmed by means of discontinuous density gradients79. Reported cryostorage-dependent alterations included a significant increase of cytosolic glycerol as a consequence of the glycerolisation step; this was not fully reverted upon thawing/washing for deglycerolisation of the units. It was, therefore, concluded that it might be profitable to replace glycerol with non-penetrating cryoprotectants, as being studied currently79.
Despite the encouraging results, it has been wondered whether cyropreserved units would be readily available and could meet the demands in the case of calamities or disasters80. Indeed, the thawing/deglycerolisation and washing steps are rather time-consuming and, in the recent past, military and civil forces had to cope with such technical inconveniences in the case of disasters (earthquakes, terrorist attacks, wars) and look for alternate logistics to meet immediate and exceptional blood demands. In this view, cryopreservation of erythrocytes seems to be better suited to storing units from certain donors with rare blood groups (e.g. Bombay)80.
Storage solutions
Another trend in transfusion medicine involves the design and testing of alternative storage solutions81. Even though huge strides have been made over the last century in this field, biochemical and “-omics” analyses (above all, metabolomics) have given new lymph to this research endeavour81.
Despite meeting haemolysis and 24h-post-transfusion in vivo survival parameters, it is evident that not all the currently patented and issued additives perform equally well. In particular, in vitro measurements82 and “-omics” investigations83 have recently highlighted how erythrocytes can be stored better in the presence of AS-1 and AS-3, respectively, than in saline-adenine-glucose-mannitol (SAGM).
Among the most extensively investigated alternative additives, promising results have been obtained for alkaline chloride-free hypotonic additive solutions (reviewed by Hess et al.84), which appear to maintain RBC quality better than the conventional additive solutions, giving at least 2 weeks’ advantage to RBC stored in the new generation additive solutions40,84. In this context, it is worth noting that all of the currently licensed additive solutions for storage of erythrocyte concentrates have an acidic pH (~5.6–5.8), which is relevant in comparison to the normal physiological pH of 7.3 of venous blood. An acidic pH facilitates heat sterilisation of additive solutions (and anticoagulants), since glucose-containing solutions caramelise when heated at a physiological/alkaline pH. While erythrocytes stored in acidic additive solutions can buffer the pH towards physiological values during the first days of storage, such a capacity is soon exhausted given the generation of lactic acid through glycolysis. The use of chloride-free formulations (such as phosphate-adenine-glucose-guanosine-gluconate-mannitol [PAGGGM]38,40) could promote the so-called Donnan equilibrium, in that chloride anions would leave the cells and favour the intracellular diffusion of hydroxide anions, thus promoting alkalinisation of the cytosol, prolonging the activity of glycolytic enzymes and preserving ATP and DPG, albeit at the expense of erythrocyte dehydration40,85.
Although it might be difficult to prevent metabolic lesions completely, alternative solutions have been proposed to mitigate metabolic lesions (e.g. a decrease in the levels of high energy phosphate compounds) as storage progresses. Another strategy to postpone the exhaustion of high energy phosphate compounds during prolonged storage involves a rejuvenation protocol, based on supplementing packed red cells with a solution containing adenine, inosine, sodium phosphate and pyruvate86. However, from a biochemical standpoint, it is worth noting that while rejuvenation solutions are indeed effective at replenishing ATP and DPG reservoirs for a longer timespan, their effect on the rest of metabolism has yet to be assessed. This is especially important in the light of the fact that several phenomena involving the protein fraction (fragmentation, oxidation) could result in the accumulation of irreversible lesions and thus theoretically alter the effectiveness of rejuvenation treatment.
Given the central role of oxidative stress in triggering storage lesions, researchers over the years have proposed the formulation of alternative storage solutions that include high doses of anti-oxidants87–89. Basically, these formulations would exploit anti-oxidant vitamins (E and C - ascorbic acid) and glutathione loading87–89. Such formulations would thus boost glutathione homeostasis and restore redox homeostasis. Of note, laboratory investigations of such formulations have highlighted the potential benefits in terms of preserved redox homeostasis, reduced membrane fragility, preserved morphology and improved post-transfusion recovery (in a mouse model of transfusion)87. However, conflicting effects have been reported in terms of the indirect consequences on energy metabolism, since some anti-oxidant compounds, such as ascorbate (dehydroascorbate, the oxidized form), would compete with the glucose transporter GLUT for uptake and would, therefore, theoretically impair energy metabolism to some extent89.
Others have proposed recommending donors to increase their dietary intake of anti-oxidant vitamins before donating blood.
Anaerobic storage
Energy and redox metabolism in RBC are intimately intertwined. As briefly described in the paragraphs above, oxygen-dependent metabolic modulation of RBC results in deoxygenation, promoting glycolysis via deoxyhaemoglobin binding to the cytosolic domain of band 3 and subsequent release and activation of glycolytic enzymes thereby bound/inhibited52. In other terms, erythrocyte deoxygenation would theoretically promote energy metabolism and thus fuel the generation of high energy phosphate compounds. At the same time, oxygen removal would eliminate the main substrate for the generation of ROS, and would thus help to tackle oxidative stress90.
On this background, Yoshida’s group proposed and patented a strategy for large-scale deoxygenation of erythrocyte concentrates, and demonstrated how anaerobic storage of RBC could promote erythrocyte survival (less haemolysis) and improve ATP and DPG conservation by the end of the storage period90. By coupling this approach to alternative additive formulations (either low or high pH) and rejuvenation treatments, the authors managed to extend the shelf-life of packed red cells to as long as 12 weeks90. Five clinical trials over the last 14 years have confirmed the potential benefits deriving from anaerobic storage, while further clinical results are awaited in the near future90. Notably, Yoshida’s apparatus offered the potential for scaling up and immediate integration in the cell processing workflow in transfusion services. However, in all five studies from Yoshida’s group, SO2 of Hb was reduced to below 4% by an experimental gas exchange protocol90.
In the light of these results, we also set up in-house deoxygenation equipment for laboratory experiments on possible complete anaerobic storage (testing with dissolved oxygen sensors by tryptophan fluorescence quenching indicated pO2 levels below 1 ppb [<0.0021 mmHg], below the instrumental limit of detection)91. We observed that storing erythrocytes under anaerobic conditions resulted in less haemolysis and better preservation of morphology91, together with reduced osmotic fragility and membrane protein fragmentation/vesiculation91,92. Metabolomic studies partly confirmed the improvements in terms of ATP and DPG preservation, although at the expense of glutathione homeostasis at the moment of the analysis93. This was probably due to the impaired potential to replenish reduced/oxidized glutathione via NADPH, since deoxygenation impairs the oxygen-dependent metabolic shift towards the PPP and thus NADPH generation, thereby potentially exposing deoxygenated erythrocytes to oxidative stress when returned to the normoxic condition after prolonged deoxygenation93,94. Furthermore, we detected an increased accumulation of metabolites involved in nitrogen metabolism,93 which would underlie deoxygenation-dependent nitric oxide generation (and thus reactive nitrogen species upon reoxygenation), as the likely result of a biological mechanism involving erythrocyte endothelial nitric oxide synthase and arginase in the promotion of vasodilation in response to hypoxia56.
Haemoglobin-based oxygen carriers
Therapeutic blood transfusions are administered with two main goals: fast recovery of oxygen delivery to organs (especially the brain) and volaemia, in order to stabilise the blood pressure and thereby guarantee efficient blood flow. While the issue of volaemia can be addressed to some extent by means of blood expanders, such as colloid- and crystalloid-based solutions, there is no currently approved alternative to red cells to cope with the necessity to sustain oxygen supply to the brain and peripheral tissues. One of the long sought-after alternatives in this regard is represented by artificial blood substitutes, also referred to as haemoglobin-based oxygen carriers (HBOC)95. The interested reader is referred to the articles by Mozzarelli et al.95 and Weiskopf and Silverman96 for further information about the different HBOC proposed over the years, as well as their potential benefits and pitfalls as therapeutic agents.
Stem cell-derived, ex-vivo generated red blood cells
The last frontier in transfusion medicine is the possibility of expanding ex vivo stem cells and inducing them to differentiate into mature erythrocytes for transfusion purposes (extensively reviewed in a thematic issue of Stem Cells International97,98). Revolutionary research in stem cell biology has recently demonstrated the possibility of generating a potentially unlimited supply of stem cells by epigenetic/genetic treatments of somatic cells (T cells, fibroblasts, others) from any individual, with successful proof-of-principle trials98. Despite these early successes, reprogramming technology is still under development. Therefore, RBC expanded ex vivo from currently discarded primary stem cell sources (buffy coats produced during blood manufacturing processes and low-volume umbilical cord blood) are being considered for first-in-man studies. Extensive proteomic investigations on umbilical cord blood CD34+ hematopoietic stem cells have been performed during the last few years99. Finally, precious insights have recently been obtained through the application of transcriptomic and phospho-proteomic analyses of mature erythroblasts expanded in vitro from normal donors and from patients with polycythemia vera100.
Concluding remarks
In the present review, we have briefly summarised the combined efforts of the international community in the field of transfusion medicine, focusing particularly on RBC storage and alternative storage strategies for erythrocyte concentrates and drawing on the collaboration between the Italian Blood Centre and the Laboratory of Proteomics and Mass Spectrometry at Tuscia University in Viterbo (Figure 1). We hope that this eagle-eye view of the RBC storage universe has shown that the international scientific community is continuously striving to find alternative, safer, more effective and, if possible, less expensive therapeutic options, although decades of improvements have already brought about significant advances in this field.
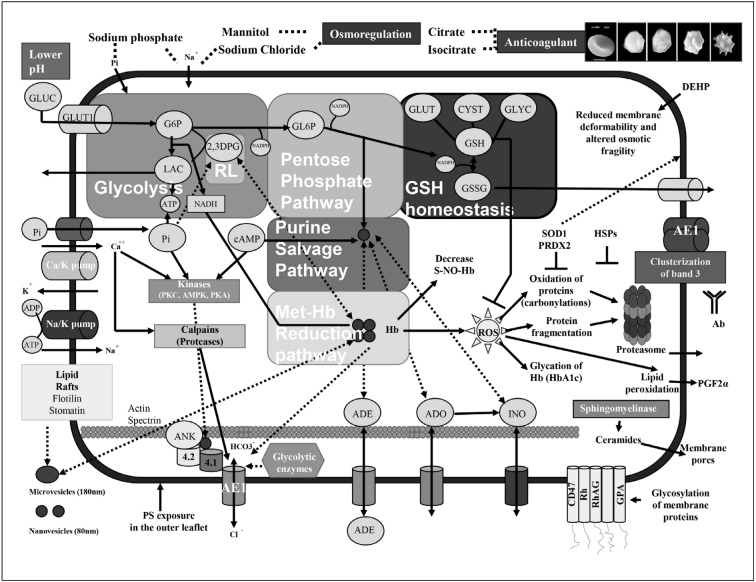
Figure 1.
The figure can be read from the upper-left corner in an anti-clockwise direction. An overview of the main biochemical changes of red blood cells (RBC) ageing in vitro under blood bank conditions.
Cation homeostasis (K+, Ca2+) is dysregulated by low temperatures and progressive depletion of high-energy phosphate reservoirs (adenosine triphosphate - ATP and 2,3-diphosphoglycerate - DPG). Glucose (additive solution) is internalised through GLUT transporters and consumed through the Emden-Meyerhof glycolytic pathway, in order to produce ATP, lactate (LAC) and promote pH lowering. Storage also results in a progressive decrease of S-nitrosothiol-haemoglobin (Hb). However, low temperatures and the progressive accumulation of oxidative stress (probably triggered by Hb-mediated Fenton reactions) promote a metabolic diversion towards the pentose phosphate pathway, in order to produce oxidized glutathione (GSSG)- reducing NADPH from glucose 6-phosphate (G6P). Pentose phosphate pathway intermediates can re-enter glycolysis or proceed towards the purine salvage pathway (also influenced by adenosine and inosine in the additive/rejuvenation solution). Alterations to calcium (Ca2+) homeostasis (and of other second messenger signalling molecules, such as cAMP and AMP) promote the activation of specific kinases (e.g. PKC, PKA, AMPK) or activate proteolytic enzymes (such as calpains) that start digesting structural and functional proteins in the cytosol and membrane, above all band 3 (AE1). Anion exchanger 1/band 3 (AE1) is responsible for the chloride shift, whereby bicarbonate (HCO3−) is exchanged for chloride (Cl−), thus modulating anion homeostasis, intracellular pH and, indirectly, Hb-oxygen affinity and thus gas exchange. Fragmentation of the cytosolic domain of AE1 (also mediated by reactive oxygen species, ROS) promotes displacement of glycolytic enzymes (thereby bound/inhibited) and structural proteins (ankyrin, ANK, band 4.2 and 4.1). Enhanced oxidation of cytosolic proteins is partly challenged by antioxidant defences (SOD1, PRDX2) and chaperone molecules (heat shock proteins, HSP), while they progressively result in the accumulation of redox modifications to proteins (carbonylation, glycation of haemoglobin [HbA1c], protein fragmentation) and lipids (lipid peroxidation, accumulation of prostaglandins in the supernatant). Alternative degradation strategies for proteins (proteasome, eventually extruded in the supernatant) and lipids (sphingomyelinase-dependent accumulation of ceramides) also play a role in this process. Progressive leaching of plasticizers (DEHP) from the plastic bag results in local accumulation in cell membranes. At the membrane level, AE1 clusters, exposure of phosphatidylserine (PS) in the outer leaflet, and lipid raft formation alter the pro-immunogenic potential of RBC. Taken together, these alterations affect membrane deformability, increase osmotic fragility and promote vesiculation, a process in which micro- and nanovesicles are shed in order to eliminate irreversibly altered proteins (including traces of glycolytic enzymes), enriched with haemoglobin and lipid raft proteins, membrane portions (also exposing common rheological antigens, such as CD47, Rh, RhAG, glycophorin A-GPA). Reprinted with permission from D’Alessandro and Zolla, Biochemistry of red cell aging in vivo and storage lesions. European Haematology Association - EHA 18 Educational Book; Haematologica 2013; 98 (Suppl 1): 389–96.
In this review, we purposely omitted those aspects related to blood safety per se101, which would have required a whole independent review, especially in the light of recently emerging concerns fostered by the minor epidemic outbreaks of West Nile virus102 and Chikungunya virus103 infections in Italy. In the next few years, National Blood Centres will have to tackle these threats before their potential spread, as a result of the additional burdens represented by global climate changes, faster and easier international travel, lifestyle modifications and sexual habits104. In this view, evaluation of pathogen inactivation strategies for stored RBC105, also with the help of “-omics” technologies, is warmly awaited.
In parallel, prospective, clinical trials will be soon completed and the results will be available for direct integration with the outcomes of laboratory investigations. Meanwhile, the scientific community is trying to provide easy-to-implement solutions that are economically sustainable in the transfusion setting, while probably promoting significant improvements in the quality of long-stored erythrocyte concentrates. One of the most promising strategies is based on vesicle removal filters and red cell washing and resuspension prior to transfusion31,106, a workflow that would prevent a broad spectrum of vesicle and pro-inflammatory lipid-related untoward effects, such as some immunogenic and inflammatory-related responses in the recipients.
Footnotes
Funding
ADA, SR and LZ are supported by the Italian National Blood Centre (National Institute of Health, Rome, Italy). ADA was supported by post-doctoral student funds from the Interuniversity Consortium for Biotechnologies.
All the Authors state that they have no conflicts of interests regarding this article.
References
- 1.Grazzini G, Ozino Caligaris A, Aprili G, et al. The new deal of the Italian Blood System. Vox Sang. 2007;93(Suppl 1):22. [Google Scholar]
- 2.Liumbruno G, D’Alessandro A, Grazzini G, Zolla L. How has proteomics informed transfusion biology so far? Crit Rev Oncol Hematol. 2010;76:153–72. doi: 10.1016/j.critrevonc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro A, Giardina B, Gevi F, et al. Clinical metabolomics: the next stage of clinical biochemistry. Blood Transfus. 2012;10(Suppl 2):s19–24. doi: 10.2450/2012.005S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zolla L, D’Alessandro A. Shaking hands with the future through omics application in transfusion medicine and clinical biochemistry. Blood Transfus. 2012;10(Suppl 2):s1–3. doi: 10.2450/2012.001S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolla L. Proteomics and transfusion medicine: the bet is open. Blood Transfus. 2010;8(Suppl 3):s1–5. doi: 10.2450/2010.001S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zolla L. Blood proteomics. Preface. J Proteomics. 2010;73:361–4. doi: 10.1016/j.jprot.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Zolla L, D’Alessandro A. Preface to the special issue: integrated omics. J Proteomics. 2012;76:3–9. doi: 10.1016/j.jprot.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Egidi MG, Rinalducci S, Marrocco C, et al. Proteomic analysis of plasma derived from platelet buffy coats during storage at room temperature. An application of ProteoMiner™ technology. Platelets. 2011;22:252–69. doi: 10.3109/09537104.2010.550348. [DOI] [PubMed] [Google Scholar]
- 9.Marrocco C, D’Alessandro A, Girelli G, Zolla L. Proteomic analysis of platelets treated with gamma irradiation versus a commercial photochemical pathogen reduction technology. Transfusion. 2013;53:1808–20. doi: 10.1111/trf.12060. [DOI] [PubMed] [Google Scholar]
- 10.Prudent M, D’Alessandro A, Cazenave JP, et al. Pathogen reduction and proteomic lesions of platelets: a transfusion medicine perspective. Transfus Med Rev. 2014;28:72–83. doi: 10.1016/j.tmrv.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 11.D’Amici GM, Blasi B, D’Alessandro A, et al. Plasma-derived clotting factor VIII: heterogeneity evaluation in the quest for potential inhibitory-antibody stimulating factors. Electrophoresis. 2011;32:2941–50. doi: 10.1002/elps.201100246. [DOI] [PubMed] [Google Scholar]
- 12.Blasi B, D’Amici GM, D’Alessandro A, Zolla L. Native analysis of plasma-derived clotting factor VIII concentrates: “sponge effect” and contaminants. Electrophoresis. 2012;33:1292–8. doi: 10.1002/elps.201100702. [DOI] [PubMed] [Google Scholar]
- 13.D’Amici GM, Timperio AM, Gevi F, et al. Recombinant clotting factor VIII concentrates: heterogeneity and high-purity evaluation. Electrophoresis. 2010;31:2730–9. doi: 10.1002/elps.201000216. [DOI] [PubMed] [Google Scholar]
- 14.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 15.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384–94. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 16.Koch CG, Figueroa PI, Li L, et al. Red blood cell storage: how long is too long? Ann Thorac Surg. 2013;96:1894–9. doi: 10.1016/j.athoracsur.2013.05.116. [DOI] [PubMed] [Google Scholar]
- 17.Liumbruno GM, Aubuchon JP. Old blood, new blood or better stored blood? Blood Transfus. 2010;8:217–9. doi: 10.2450/2010.0060-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess JR. Red cell storage: when is better not good enough? Blood Transfus. 2009;7:172–3. doi: 10.2450/2009.0110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grazzini G, Vaglio S. Red blood cell storage lesion and adverse clinical outcomes: post hoc ergo propter hoc? Blood Transfus. 2012;10(Suppl 2):s4–6. doi: 10.2450/2012.002S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen B, Matot I. Aged erythrocytes: a fine wine or sour grapes? Br J Anaesth. 2013;111:i62–70. doi: 10.1093/bja/aet405. [DOI] [PubMed] [Google Scholar]
- 21.Antonelou MH, Tzounakas VL, Velentzas AD, et al. Effects of pre-storage leukoreduction on stored red blood cells signaling: a time-course evaluation from shape to proteome. J Proteomics. 2012;76:220–38. doi: 10.1016/j.jprot.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 22.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–8. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Alessandro A, Zolla L. Biochemistry of red cell aging in vivo and storage lesions. European Haematology Association - EHA 18 Educational Book; Haematologica. 2013;98(Suppl 1):389–96. [Google Scholar]
- 24.D’Amici GM, Rinalducci S, Zolla L. Depletion of hemoglobin and carbonic anhydrase from erythrocyte cytosolic samples by preparative clear native electrophoresis. Nat Protoc. 2011;7:36–44. doi: 10.1038/nprot.2011.427. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro A, Righetti PG, Zolla L. The red blood cell proteome and interactome: an update. J Proteome Res. 2010;9:144–63. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- 26.Pallotta V, D’Alessandro A, Rinalducci S, Zolla L. Native protein complexes in the cytoplasm of red blood cells. J Proteome Res. 2013;12:3529–46. doi: 10.1021/pr400431b. [DOI] [PubMed] [Google Scholar]
- 27.Goodman SR, Daescu O, Kakhniashvili DG, Zivanic M. The proteomics and interactomics of human erythrocytes. Exp Biol Med (Maywood) 2013;238:509–18. doi: 10.1177/1535370213488474. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A, Blasi B, D’Amici GM, et al. Red blood cell subpopulations in freshly drawn blood: application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 2013;11:1–13. doi: 10.2450/2012.0164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonova G, Tung JP, Fraser JF, et al. A comprehensive ovine model of blood transfusion. Vox Sang. 2014;106:153–60. doi: 10.1111/vox.12076. [DOI] [PubMed] [Google Scholar]
- 31.Cortés-Puch I, Wang D, Sun J, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–11. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasev M, Alfano K, Chakraborty S, et al. Similar donors - similar blood? Transfusion. 2013;54(3 Pt 2):933–41. doi: 10.1111/trf.12457. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Messana I, Ferroni L, Misiti F, et al. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 38.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. PLoS One. 2013;8:e71060. doi: 10.1371/journal.pone.0071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roback JD, Josephsona CD, Wallera EK, et al. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfusion Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burger P, Korsten H, De Korte D, et al. An improved red blood cell additive solution maintains 2,3-diphosphoglycerate and adenosine triphosphate levels by an enhancing effect on phosphofructokinase activity during cold storage. Transfusion. 2010;50:2386–92. doi: 10.1111/j.1537-2995.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 41.van’t Erve TJ, Wagner BA, Martin SM, et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion. 2014;54:2055–63. doi: 10.1111/trf.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105:271–82. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51:1450–9. doi: 10.1111/j.1537-2995.2010.03026.x. [DOI] [PubMed] [Google Scholar]
- 44.Dumaswala UJ, Zhuo L, Jacobsen DW, et al. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27:1041–9. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 45.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–55. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Amici GM, Rinalducci S, Zolla L. Proteomic analysis of RBC membrane protein degradation during blood storage. J Proteome Res. 2007;6:3242–55. doi: 10.1021/pr070179d. [DOI] [PubMed] [Google Scholar]
- 47.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 48.Walpurgis K, Kohler M, Thomas A, et al. Storage-induced changes of the cytosolic red blood cell proteome analyzed by 2D DIGE and high-resolution/high-accuracy MS. Proteomics. 2012;12:3263–72. doi: 10.1002/pmic.201200280. [DOI] [PubMed] [Google Scholar]
- 49.Rinalducci S, D’Amici GM, Blasi B, Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie. 2011;93:845–53. doi: 10.1016/j.biochi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Rinalducci S, Ferru E, Blasi B, et al. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012;10:s55–62. doi: 10.2450/2012.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang E, Qadri SM, Lang F. Killing me softly - suicidal erythrocyte death. Int J Biochem Cell Biol. 2012;44:1236–43. doi: 10.1016/j.biocel.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 52.Castagnola M, Messana I, Sanna MT, Giardina B. Oxygen-linked modulation of erythrocyte metabolism: state of the art. Blood Transfus. 2010;8:53–8. doi: 10.2450/2010.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sparrow RL, Veale MF, Healey G, Payne KA. Red blood cell (RBC) age at collection and storage influences RBC membrane-associated carbohydrates and lectin binding. Transfusion. 2007;47:966–8. doi: 10.1111/j.1537-2995.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 54.D’Alessandro A, Mirasole C, Zolla L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a MALDI-TOF mass-spectrometry-based investigation. Vox Sang. 2013;105:177–80. doi: 10.1111/vox.12029. [DOI] [PubMed] [Google Scholar]
- 55.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 56.Cortese-Krott MM, Kelm M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol. 2014;2:251–8. doi: 10.1016/j.redox.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timperio AM, Mirasole C, D’Alessandro A, Zolla L. Red blood cell lipidomics analysis through HPLC-ESI-qTOF: application to red blood cell storage. J Integrated Omics. 2013;3:11–24. [Google Scholar]
- 58.Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–67. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 59.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Y, Chen J, Cui T, et al. Characterization of red blood cell deformability change during blood storage. Lab Chip. 2013;14:577–83. doi: 10.1039/c3lc51151k. [DOI] [PubMed] [Google Scholar]
- 61.Bosman GJ, Cluitmans JC, Groenen YA, et al. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion. 2011;51:1072–8. doi: 10.1111/j.1537-2995.2010.02929.x. [DOI] [PubMed] [Google Scholar]
- 62.Dumont LJ, Baker S, Dumont DF, et al. Exploratory in vitro study of red blood cell storage containers formulated with an alternative plasticizer. Transfusion. 2012;52:1439–45. doi: 10.1111/j.1537-2995.2011.03506.x. [DOI] [PubMed] [Google Scholar]
- 63.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–20. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 64.Anniss AM, Glenister KM, Killian JJ, Sparrow RL. Proteomic analysis of supernatants of stored red blood cell products. Transfusion. 2005;45:1426–33. doi: 10.1111/j.1537-2995.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 65.Dzieciatkowska M, Silliman CC, Moore EE, et al. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013;105:210–8. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosman GJ, Lasonder E, Groenen-Döpp YA, et al. The proteome of erythrocyte-derived microparticles from plasma: new clues for erythrocyte aging and vesiculation. J Proteomics. 2012;76:203–10. doi: 10.1016/j.jprot.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 67.Canellini G, Rubin O, Delobel J, et al. Red blood cell microparticles and blood group antigens: an analysis by flow cytometry. Blood Transfus. 2012;10(Suppl 2):s39–45. doi: 10.2450/2012.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 69.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76:181–93. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Willekens FL, Werre JM, Groenen-Döpp YA, et al. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 71.Veale MF, Healey G, Sparrow RL. Longer storage of red blood cells is associated with increased in vitro erythrophagocytosis. Vox Sang. 2014;106:219–26. doi: 10.1111/vox.12095. [DOI] [PubMed] [Google Scholar]
- 72.Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. doi: 10.3389/fphys.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 74.Lesch A, Cortés-Salazar F, Prudent M, et al. Large scale inkjet-printing of carbon nanotubes electrodes for antioxidant assays in blood bags. J Electroanalytical Chem. 2014;717–718:61–8. [Google Scholar]
- 75.Marrocco C, Pallotta V, D’Alessandro A, et al. Red blood cell populations and membrane levels of peroxiredoxin 2 as candidate biomarkers to reveal blood doping. Blood Transfus. 2012;10(Suppl 2):s71–7. doi: 10.2450/2012.011S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pottgiesser T, Schumacher YO. Current strategies of blood doping detection. Anal Bioanal Chem. 2013;405:9625–39. doi: 10.1007/s00216-013-7270-x. [DOI] [PubMed] [Google Scholar]
- 77.Blake JT, Hardy M, Delage G, Myhal G. Déjà-vu all over again: using simulation to evaluate the impact of shorter shelf life for red blood cells at Héma-Québec. Transfusion. 2013;53:1544–58. doi: 10.1111/j.1537-2995.2012.03947.x. [DOI] [PubMed] [Google Scholar]
- 78.Valeri CR, Srey R, Tilahun D, Ragno G. The in vitro quality of red blood cells frozen with 40 percent (wt/vol) glycerol at −80 degrees C for 14 years, deglycerolized with the Haemonetics ACP 215, and stored at 4 degrees C in additive solution-1 or additive solution-3 for up to 3 weeks. Transfusion. 2004;44:990–5. doi: 10.1111/j.1537-2995.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- 79.Pallotta V, D’Amici GM, D’Alessandro A, et al. Red blood cell processing for cryopreservation: from fresh blood to deglycerolization. Blood Cells Mol Dis. 2012;48:226–32. doi: 10.1016/j.bcmd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Whitsett C, Vaglio S, Grazzini G. Alternative blood products and clinical needs in transfusion medicine. Stem Cells Int. 2012;2012:639561. doi: 10.1155/2012/639561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10(Suppl 2):s7–11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sparrow RL, Sran A, Healey G, et al. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2014;54:560–8. doi: 10.1111/trf.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D’Amici GM, Mirasole C, D’Alessandro A, et al. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012;10(Suppl 2):s46–54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hess JR, Hill HR, Oliver CK, et al. Alkaline CPD and the preservation of RBC 2,3-DPG. Transfusion. 2002;42:747–52. doi: 10.1046/j.1537-2995.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- 85.Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion. 2011;51:25S–33S. doi: 10.1111/j.1537-2995.2010.02960.x. [DOI] [PubMed] [Google Scholar]
- 86.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2011;51:1574–9. doi: 10.1111/j.1537-2995.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 87.Stowell SR, Smith NH, Zimring JC, et al. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion. 2013;53:2248–57. doi: 10.1111/trf.12106. [DOI] [PubMed] [Google Scholar]
- 88.Pallotta V, Naro F, Gevi F, et al. Supplementation of anti-oxidants in leucofiltered erythrocyte concentrates: assessment of morphological changes through scanning electron microscopy. Blood Transfus. 2014;12:421–4. doi: 10.2450/2014.0272-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pallotta V, Gevi F, D’Alessandro A, Zolla L. Red blood cell storage with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus. 2014;12:376–87. doi: 10.2450/2014.0266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zolla L, D’Alessandro A. An efficient apparatus for rapid deoxygenation of erythrocyte concentrates for alternative banking strategies. J Blood Transfus. 2013;2013:896537. doi: 10.1155/2013/896537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Longo V, D’Alessandro A, Zolla L. Deoxygenation of leukofiltered erythrocyte concentrates preserves proteome stability during storage in the blood bank. Blood Transfusion. 2014;12:599–604. doi: 10.2450/2014.0335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst. 2013;9:1196–209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 94.Rogers SC, Said A, Corcuera D, et al. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009;23:3159–70. doi: 10.1096/fj.09-130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mozzarelli A, Ronda L, Faggiano S, et al. Haemoglobin-based oxygen carriers: research and reality towards an alternative to blood transfusions. Blood Transfus. 2010;8(Suppl 3):s59–68. doi: 10.2450/2010.010S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiskopf RB, Silverman TA. Balancing potential risks and benefits of hemoglobin-based oxygen carriers. Transfusion. 2013;53:2327–33. doi: 10.1111/trf.12339. [DOI] [PubMed] [Google Scholar]
- 97.Zeuner A, Martelli F, Vaglio S, et al. Concise review: stem cell-derived erythrocytes as upcoming players in blood transfusion. Stem Cells. 2012;30:1587–96. doi: 10.1002/stem.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Migliaccio AR, Grazzini G, Hillyer CD. Ex vivo generated red cells as transfusion products. Stem Cells Int. 2012;2012:615412. doi: 10.1155/2012/615412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Alessandro A, Grazzini G, Giardina B, Zolla L. In silico analyses of proteomic data suggest a role for heat shock proteins in umbilical cord blood hematopoietic stem cells. Stem Cell Rev. 2010;6:532–47. doi: 10.1007/s12015-010-9180-z. [DOI] [PubMed] [Google Scholar]
- 100.Hricik T, Federici G, Zeuner A, et al. Transcriptomic and phospho-proteomic analyzes of erythroblasts expanded in vitro from normal donors and from patients with polycythemia vera. Am J Hematol. 2013;88:723–9. doi: 10.1002/ajh.23487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hess JR, Grazzini G. Blood proteomics and transfusion safety. J Proteomics. 2010;73:365–7. doi: 10.1016/j.jprot.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 102.Pupella S, Pisani G, Cristiano K, et al. West Nile virus in the transfusion setting with a special focus on Italian preventive measures adopted in 2008–2012 and their impact on blood safety. Blood Transfus. 2013;11:563–74. doi: 10.2450/2013.0077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liumbruno GM, Calteri D, Petropulacos K, et al. The Chikungunya epidemic in Italy and its repercussion on the blood system. Blood Transfus. 2008;6:199–210. doi: 10.2450/2008.0016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suligoi B, Pupella S, Regine V, et al. Changing blood donor screening criteria from permanent deferral for men who have sex with men to individual sexual risk assessment: no evidence of a significant impact on the human immunodeficiency virus epidemic in Italy. Blood Transfus. 2013;11:441–8. doi: 10.2450/2013.0162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henschler R, Seifried E, Mufti N. Development of the S-303 pathogen inactivation technology for red blood cell concentrates. Transfus Med Hemother. 2011;38:33–42. doi: 10.1159/000324458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silliman CC, Kelher MR, Khan SY, et al. Experimental pre-storage filtration removes antibodies and decreases lipid bioactivity accumulation in RBC supernatants mitigating TRALI in an animal model. Blood. 2014;123:3488–95. doi: 10.1182/blood-2013-10-532424. [DOI] [PMC free article] [PubMed] [Google Scholar]