Introduction
Inherited epidermolysis bullosa (EB) consists of a number of genetically heterogeneous skin diseases characterised by defects in the synthesis of proteins involved in the adhesion of the epidermis to the dermis1. The common clinical manifestations of EB are mechanical fragility of the skin, blister formation, and abnormal wound healing, but the severity of the skin lesions varies depending on the distinct type of the disease. Junctional EB and dystrophic EB (DEB), the most severe types of EB diseases, usually cause chronic pain and discomfort that can significantly reduce the quality of life of affected patients2. In addition, non-healing wounds can cause severe systemic infections, malnutrition, and growth inhibition, and may promote carcinogenesis in some cases3. Wound care is, therefore, of critical importance in the management of EB4. However, the current treatments for EB, including the most recently suggested approaches, are not always effective or satisfactory.
It has been reported that several autologous and allogeneic platelet preparations -in particular a blood component termed “platelet gel”, which has been traditionally obtained from adult blood platelets- are useful for the treatment of chronic wounds5–7. In this study we performed a pilot evaluation of the safety and effectiveness of platelet gel derived from cord blood (CBPG)8 -a recently described allogeneic biological product which has been regulated as a blood component in Italy- for the treatment of skin lesions in three children with recessive DEB.
Case reports
Three children (aged 7–14 years) with recessive DEB skin lesions were included in this pilot study. Recessive DEB was diagnosed on the basis of clinical, histological and/or molecular findings (patient 1: COL7A1 c.497dup, c.4827_4834del; patient 2 diagnosed only on the basis of electron microscopy findings; patient 3: COL7A1 c.497dup, c.4991G>C) in the Epidemolysis Bullosa Referral Centre of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy. Written informed consent was signed by the patients’ parents prior to the study. The study protocol was approved by the Institutional Ethics Committee, and was conducted in accordance with the standards of Good Clinical Practice for trials of medicinal products in humans.
In each patient, two skin lesions (3.6±0.9×2.6±1.1 cm), excluding those from sites subject to frequent friction and repeated trauma, were randomly selected for the investigation. Punch-biopsies from the edges of the selected skin lesions were examined to ensure that the lesions had not undergone neoplastic transformation. One lesion (lesion A) was treated with a weekly application of CBPG in sterile conditions for 3 weeks and a control lesion (lesion B) was managed with standardised medication with non-biological foam dressing of the last generation.
Cord blood (CB) was collected into sterile plastic bags containing 29 mL citrate-phosphate-dextrose (Macopharma, Mauvaux, France) from healthy mothers participating in the voluntary CB donation programme of Milan Cord Blood Bank, a public CB bank belonging to the Italian Cord Blood Network. Mothers participating in this programme were asked to consent to the collection, storage, use and distribution of cord blood for allogeneic haematopoietic transplantation or, if the unit did not comply with a minimum cellularity threshold value which would qualify it for storage in the cryopreserved haematopoietic transplant inventory, for the production and clinical use of CBPG. All mothers donating the CB units used in this study consented to either use.
CB units were collected and transported at controlled temperature to the CB bank according to standard procedures. All CB samples were negative for syphilis, human immunodeficiency virus, hepatitis C virus and hepatitis B virus (both by serological tests and nucleic acid testing).
CB samples containing ≥1.5×109 nucleated cells were processed and deposited in the haematopoietic transplant inventory of the Milan Cord Blood Bank. CBPG was produced from CB samples ≥50 mL (<1.5×109 nucleated cells and ≥150×109 L platelets) collected within the preceding 48 hours8,9. Briefly, CB was aseptically transferred by sterile docking (TSCD-II, Terumo, Tokyo, Japan) into a 150 mL empty plastic bag (Macopharma) that was connected to two empty plastic bags (100 mL) (Fresenius Kabi Italia, Isola della Scala, Verona, Italy) and was centrifuged at 220 g (800 rpm) for 10 minutes in a Heraeus Cryofuge 6000i centrifuge (Kendro Laboratory Products, Hanau, Germany). The platelet-rich plasma (PRP) was slowly transferred into one of the empty satellite bags using a manual plasma extractor. The tube connecting the primary and the satellite bags was sealed and the primary bag containing the red blood cells was discarded. After gentle resuspension of PRP, platelets were counted. The PRP was then centrifuged at 2,450 rpm (2,000 g) for 15 minutes and the supernatant platelet-poor plasma was transferred into the empty satellite bag. An appropriate volume of supernatant was kept to ensure a final concentration of platelets (1.5–2.0×109/mL) in the CB platelet concentrate (CBPC). The supernatant volume ensuring the above amount of target platelets in CBPC was determined on the basis of the following formulae:
Final CBPC volumes within the predetermined range were set by weight on a laboratory scale.
After resuspension of platelets in the CBPC at room temperature for 60 minutes, an automated complete blood count was performed on a sample collected from a segment. In parallel, a screening test for aerobic and anaerobic bacteria (BacT/Alert, BioMerieux, Marci-l’Etoile, France) was performed on a 4 mL sample aseptically collected from the bag of platelet-poor plasma. Finally, the CBPC was transferred into a 20 mL bag (Biomed, Modena, Italy) with sterile docking and cryopreserved at −80 °C in a mechanical freezer until use.
About 2 hours before the planned therapeutic use, the CBPC samples were thawed in a dry thawing device at 37 °C. Units were selected randomly from the available production, with no donor-recipient matching requirement for ABO, Rh, HLA and platelet-specific antigens. The freezing/thawing procedure resulted in disruption of the intact platelets and formation of a CB platelet lysate. Gelification was induced within 20–30 minutes of the addition of batroxobin (Plateltex, Praha, Czeck Republic), according to the manufacturer’s instructions. The CBPG was carefully transferred to the patient’s bedside where the bag was disinfected with surgical betadine. CBPG was released from the bag by cutting one side of the bag with sterile scissors, applied to the skin ulcer by a trained dermatologist, and fixed with a paraffin gauze with a small-size mesh and a bandage. The paraffin gauze was completely dry 1 week later, which could have been a cause of new skin lesions by scratching. A foam-dressing frame was, therefore, used to support the gauze and prevent its contact with the skin. The patients’ activities were not restricted after application of the CBPG. The control lesion was simultaneously treated with latest generation, non-biological foam dressing (Mepilex, Molnlycke Health Care, Gothenburg, Sweden) and was renewed daily at home. Spectrum-adjusted antibiotics were not applied prior to or throughout the entire treatment of the two lesions nor was debridement performed at any time.
Skin lesions were evaluated clinically once a week during the 3-week treatment period and further monitored once a week for 4 weeks after the CBPG treatment. Moreover, morphological changes of skin lesions were documented photographically and adverse effects of CBPG were recorded. After the CBPG treatment, punch-biopsies of the edges of treated and control lesions were examined to confirm that neoplastic transformation had not occurred.
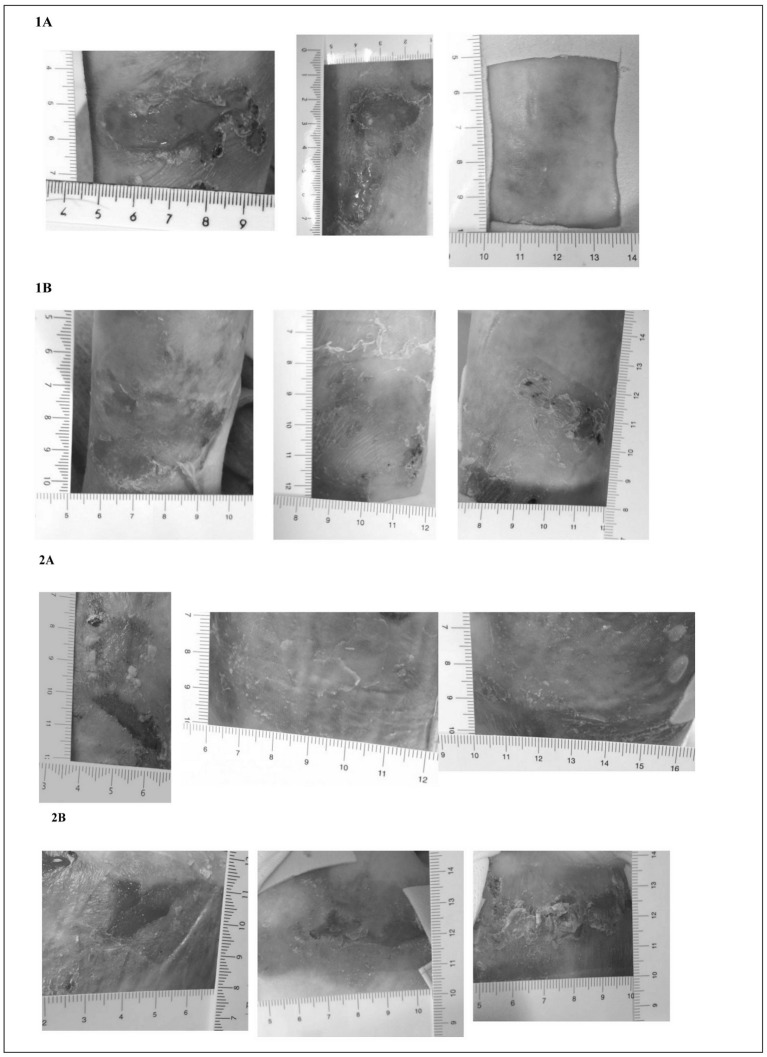
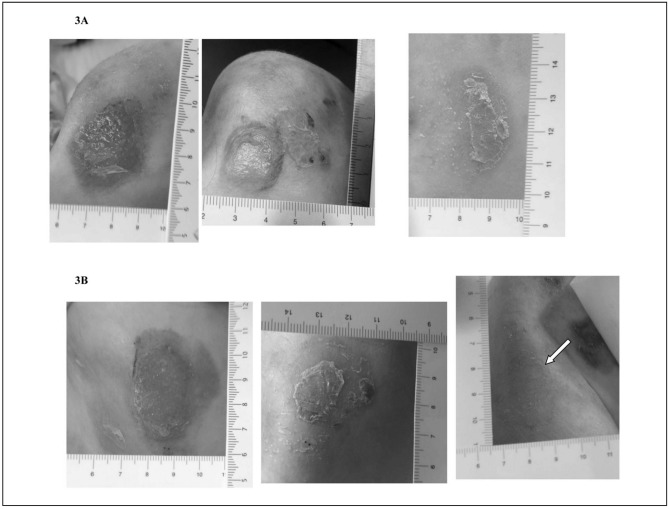
Table I summarises the characteristics and the outcomes of the EB patients. The changes of the skin lesions with CBPG treatments are illustrated in Figure 1a and 1b. Interestingly, patients 1 and 2 showed greater improvement in skin lesions treated with CBPG than in lesions treated with standard medications. In the third patient, the efficacy of CBPG treatment appeared similar to that of standard medications although the quality of skin appeared better in the lesion treated with CBPG than the lesion treated with standard medication, which partially re-opened immediately after the end of the therapy. With regards to patient 2, the child reported light itching 3–4 days after the first application of CBPG, which spontaneously resolved in 2 days; no further adverse events were reported by the children or their parents. Neoplastic transformation was not observed at the edges of the treated or control lesions before or after the study. Superinfections were not observed in lesions treated with CBPG, whereas all the control lesions showed partial re-opening with yellowish scales and scabs.
Table I.
Characteristics and outcomes of the three children with DEB treated with CBPG.
| Patient | Gender | Age (years) | Enrolment | Day 7 | Day 21 | Follow up at day 42 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lesion A (treated with CBPG) | Lesion B (standard treatments) | Lesion A (treated with CBPG) | Lesion B (standard treatments) | Lesion A (treated with CBPG) | Lesion B (standard treatments) | Lesion A (treated with CBPG) | Lesion B (standard treatments) | |||
| 1 | Male | 14 | Left arm 4.5×2 cm | Right arm 3.5×3.0 cm | Left arm 3×1.1 cm | Right arm, partial re-epithelialisation | Left arm, complete re-epithelialisation | Right arm, re-opening of the wound (1.3×1.0 cm) with scales and scabs on the edges | Left arm, healthy skin | Right arm, complete re-epithelialisation with residual erythema |
| 2 | Female | 11 | Right subscapular area 2.7×1.7 cm | Right hip 3.5×1.5 cm | Right subscapular area, complete re-epithelialisation with fine yellow scales | Right hip 2.0×1.3 cm with scabs and scales | Right subscapular area, complete re-epithelialisation | Right hip, 1.0×1.5 cm with scales and scabs | Right subscapular area, healthy skin | Right hip, 1.1×0.8 cm with erythema and scales |
| 3 | Female | 7 | Right shoulder 3.0×3.7 cm | Right lumbar region 4.5×2.0 cm | Right shoulder 2.0×2.0 cm with fine scales | Right lumbar region, complete re-epithelialisation with residual erythema, scabs and scales | Right shoulder, complete re-epithelialisation with residual erythema and fine scales | Right lumbar region, complete re-epithelialisation with residual erythema | Right shoulder, healthy skin | Right lumbar region, scabs and scales, outcome of partial re-opening of the wound |
Figure 1a.
Comparison of skin lesions treated with CBPG and standard medications in DEB patient 1 and 2.
For each patient lesion A (top) was treated with CBPG and lesion B (bottom) was treated with standard medications at enrolment (left), after 1 week of therapy (middle), and after 3 weeks of therapy (right). The arrow represents a new lesion caused by a dry dressing (see text).
Figure 1b.
Comparison of skin lesions treated with CBPG and standard medications in DEB patient 3.
For each patient lesion A (top) was treated with CBPG and lesion B (bottom) was treated with standard medications at enrolment (left), after 1 week of therapy (middle), and after 3 weeks of therapy (right). The arrow represents a new lesion caused by a dry dressing (see text).
Discussion
DEB is one of the most severe forms of EB. To the best of our knowledge, this is the first pilot study evaluating the effectiveness and safety of CBPG in the treatment of skin lesions of DEB in children. Although the number of DEB children included in this pilot study was limited, our results are encouraging because the skin lesions treated with CBPG resolved in a relatively short time and no relapses were observed by 4 weeks after the completion of the applications of CBPG. Based on our preliminary results, it is important to further study the underlying mechanism of CBPG in skin repair in EB, especially the role of growth factors secreted by CBPG in chronic wound managements.
Allogeneic platelet gel units were used in this study. Previously, autologous preparations of platelet gel were used more frequently to deliver platelet-derived growth factors to chronic wounds5–7. However, a large amount of blood is usually necessary to prepare a sufficient amount of autologous platelet gel, which may have detrimental effects on haemodynamic stability because subjects with chronic wounds often have anaemia and poor health. Allogeneic products can overcome these disadvantages of autologous platelet gel. In addition, CBPG has a higher concentration of growth factors than gel obtained from adult platelets8,9. For these reasons, we used allogeneic platelet gel derived from CB in this study.
Various new dressings have recently been developed to reduce the impact of wound management on the quality of life of EB patients. However, frequent changes of dressings and the difficult healing of some chronic wounds, especially those in areas of friction and repeated trauma, remain major challenges in the treatment of EB skin lesions10–12. CBPG releases a number of growth factors such as platelet-derived growth factor-BB and -AB, transforming growth factor-β1 and -β2, insulin-like growth factor-I, epidermal growth factor, fibroblast growth factor-b, and vascular endothelial growth factor, which can modulate cell proliferation and differentiation as well as accelerate keratinocyte migration from the wound edge with the generation of intact epithelium8,9. The role of these growth factors can explain the effectiveness of CBPG in the treatment of DEB skin lesions in our study. In addition, platelet materials have antimicrobial activity against some bacteria of the skin flora and clinical studies showed that platelet materials prevented infections in wounds13,14. In our study, skin lesions treated with CBPG were improved as early as the first week of treatment and superinfections were not observed, differently from the control lesions that all showed partial re-opening with yellowish scales and scabs that are frequently evidence of skin superinfection. In addition, skin lesions treated with CBPG healed constantly, whereas control lesions exhibited clinical regression. Furthermore, no relapses were observed in skin lesions treated with CBPG and the healed skin appeared to be healthier with a well-structured corneal layer than skin lesions treated with standard medications. Given that continuous tissue stress is a major first risk factor of skin cancer, CBPG treatment may prevent the development of skin cancer by improving skin lesions2–4. Further studies on intractable skin lesions with a longer follow-up would be useful to confirm our preliminary findings.
Regarding safety, CBPG was well tolerated and no significant adverse events were reported by three DEB patients although one patient developed a light itch. Slight changes of gel medications (e.g., every 3–4 days) may be able to prevent dryness of the dressings, which could relieve itching and avoid scratching. Therefore, the development of devices to apply CBPG that can be used by EB patients and their parents at home appears the future for the extensive use of CBPG in EB patients.
It has been hypothesised that the severity, extent, and especially persistence of skin lesions in EB are associated with the risk of developing skin cancer, which starts at the end of the second decade1. In support of this hypothesis, Smoller et al. identified growth-activated keratinocytes in some biopsies from chronic skin ulcers of EB patients based on the observation of intense suprabasal expression of K16, involucrin, and filaggrin15. These observations could be associated with carcinogenesis. While skin cancers are rare in children, DEB patients in this study were still subjected to lesion biopsies at the start and end of the trial to exclude the presence of any keratinocyte transformation.
In conclusion, this pilot study reveals that CPBG is a promising and safe option for the treatment of EB skin lesions and highlights the need for further controlled larger studies to confirm our preliminary findings and to expand the use of CBPG in chronic wound management.
Acknowledgements
The Authors would like to thank Debra Italy Onlus, the staff of the Milan Cord Blood Bank and the National Blood Centre, National Institute of Health, (Rome, Italy) for their support of the project.
Footnotes
Funding and resources
This study was supported by grants from the Italian Ministry of Health (Ricerca Finalizzata RF-2009-1549214, Ricerca Corrente 850/01), Debra Italy, and Amici del Bambino Malato Onlus.
Authorship contributions
GT co-designed the study, participated in the enrolment and follow-up of the children, and assisted with data interpretation and manuscript writing; SG enrolled and followed-up the children and critically revised the study design and the manuscript; LP participated in follow-up of the children and drafted the manuscript; MM conducted the production of cord blood-derived platelet gel and critically revised the study design and manuscript; NG participated in the preparation of cord blood-derived platelet gel; FM participated in the enrolment and follow-up of the children; PR co-designed the study, participated in the preparation of cord blood-derived platelet gel and assisted with data interpretation and manuscript writing; SE co-designed the study, supervised the enrolment and follow-up of the children and wrote the manuscript. All of the Authors read and approved the final version of the manuscript.
Disclosure of conflicts of interest
PR and NG are co-inventors of patent US 2011/0123503 A1: “Platelet Fraction Deriving From Placental Blood”. None of the other Authors has any commercial or other relationship that might raise conflicts of interest.
References
- 1.Fine JD, Bruckner-Tuderman L, Eady RA, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103–26. doi: 10.1016/j.jaad.2014.01.903. [DOI] [PubMed] [Google Scholar]
- 2.Grocott P, Blackwell R, Weir H, Pillay E. Living in dressings and bandages: findings from workshops with people with epidermolysis bullosa. Int Wound J. 2013;10:274–84. doi: 10.1111/j.1742-481X.2012.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg GI, Eisen AZ, Bauer EA. Tissue stress and tumor promotion. Possible relevance to epidermolysis bullosa. Arch Dermatol. 1988;124:737–41. [PubMed] [Google Scholar]
- 4.Hsu CK, Wang SP, Lee JY, McGrath JA. Treatment of hereditary epidermolysis bullosa: updates and future prospects. Am J Clin Dermatol. 2014;15:1–6. doi: 10.1007/s40257-013-0059-z. [DOI] [PubMed] [Google Scholar]
- 5.Knighton DR, Ciresi KF, Fiegel VD, et al. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF) Ann Surg. 1986;204:322–30. doi: 10.1097/00000658-198609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knighton DR, Ciresi K, Fiegel VD, et al. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet. 1990;170:56–60. [PubMed] [Google Scholar]
- 7.Mishra A, Woodall J, Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–25. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Parazzi V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets. 2010;21:549–54. doi: 10.3109/09537104.2010.514626. [DOI] [PubMed] [Google Scholar]
- 9.Greppi N, Mazzucco L, Galetti G, et al. Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals. 2011;39:73–80. doi: 10.1016/j.biologicals.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Wollina U, Konrad H, Fisher T, et al. Recessive epidermolysis bullosa dystrophicans (Hallopeau-Siemens) - improvement of wound healing by autologous epidermal grafts on an esterified hyaluronic acid membrane. J Dermatol. 2001;28:217–20. doi: 10.1111/j.1346-8138.2001.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 11.Pasmooij AMG, Jonkman MF, Uitto J. Revertant mosaicism in heritable skin diseases: mechanisms of natural gene therapy. Discov Med. 2012;14:167–79. [PubMed] [Google Scholar]
- 12.Woodley DT, Wang X, Amir M, et al. Intravenously injected recombinant human type VII collagen homes to skin wounds and restores skin integrity of dystrophic epidermolysis bullosa. J Invest Dermatol. 2013;133:1910–3. doi: 10.1038/jid.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011;11:e38. [PMC free article] [PubMed] [Google Scholar]
- 14.Burnouf T, Chou ML, Wu YW, et al. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion. 2013;53:138–46. doi: 10.1111/j.1537-2995.2012.03668.x. [DOI] [PubMed] [Google Scholar]
- 15.Smoller BA, McNutt NS, Carter DM, et al. Recessive dystrophic epidermolysis bullosa skin displays a chronic growth-activated immunophenotype. Implications for carcinogenesis. Arch Dermatol. 1990;126:78–83. [PubMed] [Google Scholar]