Figure 3.
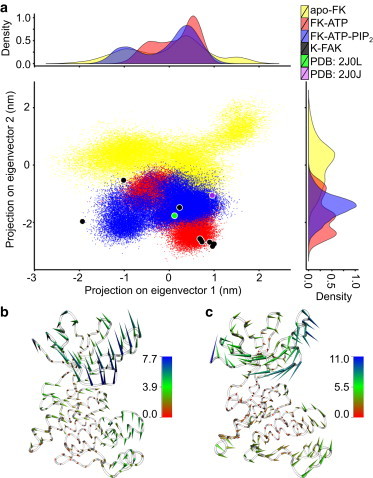
Conformational changes upon ATP/PIP2 binding measured by principal component analysis (PCA). (a) apo-FK (yellow), FK-ATP (red), FK-ATP-PIP2 (blue) during MD simulations, and crystal structures of staurosporine-bound FK-FAK (violet, PDB 2J0J), the ANP-bound K-FAK (green, PDB 2J0L), and apo K-FAK (black) were projected on the first two eigenvectors obtained from a PCA of the fluctuations of apo-FK Cα atoms. Distributions of the three states along the two apo eigenvectors are also shown. (b and c) Porcupine plot of the FAK kinase domain, illustrating the motion along the first (b) and second (c) eigenvectors (EV) obtained from PCA. Coloring is according to the deviation in eigenvalue between the two extreme structures along the respective EV. EV1 (b) describes an opening, EV2 (c) a twisting motion. To see this figure in color, go online.
