Figure 2.
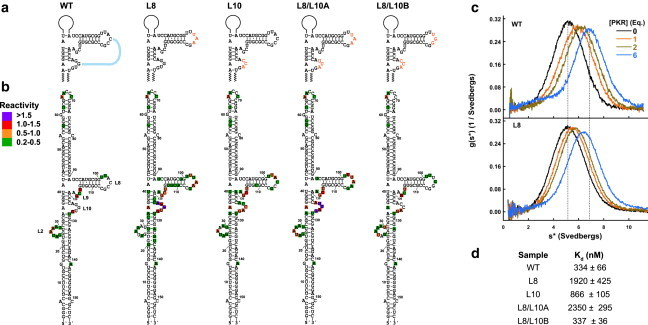
Effect of central domain mutations on VAI structure and PKR binding. (a) Secondary structure of VAI indicating central domain mutations. (b) SHAPE reactivity of central domain mutations. Reactivities were quantitated using SAFA and normalized to the band corresponding to A65. The reactivities are indicated in color scale indicated in the legend. (c) Sedimentation velocity titration of PKR binding to WT (top) and L8 (bottom) VAI. The data were processed using DCDT+ (32) to produce normalized g(s∗) sedimentation velocity distribution functions. The shift in the peak maximum upon addition of 6 eq. of PKR to WT VAI is indicated by the dotted lines. The magnitude of the shift is decreased for L8 because of a reduced binding affinity. (d) Dissociation constants for PKR interaction with central domain mutants determined by global analysis using SEDANAL (33). To see this figure in color, go online.
