Figure 2. Triple-helical peptidase activity of the ectodomain of MT1-MMP impaired by alanine substitutions at the THP binding site of its HPX domain.
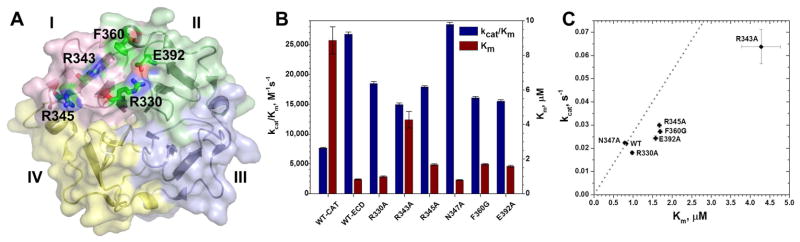
(A) Residues that impair triple-helical peptidase activity when mutated are shown with sticks and labels.
(B) The catalytic efficiency kcat/Km and Michaelis constant Km of the triple-helical peptidase activity towards fTHP-15 by the variants of the ectodomain (catalytic through HPX domains) of MT1-MMP at 27 °C, pH 7.5 are from fits of two progress curves (Palmier and Van Doren, 2007). The uncertainties plotted are three-fold the fitting errors.
(C) Catalytic turnover kcat is plotted versus Km for THP hydrolysis. Variants falling to the right of the trend line through the WT point are decreased in kcat/Km of the triple-helical peptidase activity.
