Abstract
Sex chromosomes have been studied in many plant and animal species. However, few species are suitable as models to study the evolutionary histories of sex chromosomes. We previously demonstrated that papaya (Carica papaya) (2n = 2x = 18), a fruit tree in the family Caricaceae, contains recently emerged but cytologically heteromorphic X/Y chromosomes. We have been intrigued by the possible presence and evolution of sex chromosomes in other dioecious Caricaceae species. We selected a set of 22 bacterial artificial chromosome (BAC) clones that are distributed along the papaya X/Y chromosomes. These BACs were mapped to the meiotic pachytene chromosomes of Vasconcellea parviflora (2n = 2x = 18), a species that diverged from papaya ∼27 million years ago. We demonstrate that V. parviflora contains a pair of heteromorphic X/Y chromosomes that are homologous to the papaya X/Y chromosomes. The comparative mapping results revealed that the male-specific regions of the Y chromosomes (MSYs) probably initiated near the centromere of the Y chromosomes in both species. The two MSYs, however, shared only a small chromosomal domain near the centromere in otherwise rearranged chromosomes. The V. parviflora MSY expanded toward the short arm of the chromosome, whereas the papaya MSY expanded in the opposite direction. Most BACs mapped to papaya MSY were not located in V. parviflora MSY, revealing different DNA compositions in the two MSYs. These results suggest that mutation of gene(s) in the centromeric region may have triggered sex chromosome evolution in these plant species.
Keywords: FISH, centromere, heterochromatin, sex chromosome
HETEROMORPHIC sex chromosomes (X/Y, Z/W) evolved from pairs of autosomes (Ming et al. 2011; Bachtrog 2013; Charlesworth 2013). It is generally accepted that sex chromosome evolution is initiated by the emergence of a sex-determining locus. Suppression of recombination surrounding the sex-determining locus, which favored the linkage of the sex-determining alleles with sexually antagonistic alleles, caused the decay of the Y/W chromosomes and resulted in a pair of heteromorphic sex chromosomes (Ming et al. 2011; Bachtrog 2013; Charlesworth 2013). Sex chromosomes evolved independently in different eukaryotic lineages. The mammalian X chromosome and the bird Z chromosome evolved from different portions of the ancestral genome (Bellott et al. 2010; Cortez et al. 2014). Interestingly, all therian mammals have homologous XY systems and all birds have homologous ZW systems (Graves 2008). Thus, these sex chromosomes, surprisingly, appear to have evolved only once in each of these two major evolutionary clades.
Compared to mammals and birds, sex chromosomes have emerged more frequently in several other eukaryotic lineages, including plants. Sex chromosomes have been reported in at least 48 plant species across 20 different families, including both X/Y and Z/W systems (Ming et al. 2011; Kumar et al. 2014). Sex chromosomes in some plant species were identified using genetic mapping-based approaches. However, cytogenetic data are not available from most of these species. Thus, it is unclear whether heteromorphic chromosomes have already developed in these species, such as asparagus (Asparagus officinalis) (Telgmann-Rauber et al. 2007), Populus (Populus trichocarpa) (Yin et al. 2008), and spinach (Spinacia oleracea) (Yamamoto et al. 2014). Heteromorphic sex chromosomes were revealed cytologically in several plant species (Yamato et al. 2007; Zhang et al. 2008; Sousa et al. 2013), including, Silene latifolia, which is one of the best-studied model plant species with well-differentiated X/Y chromosomes (Vyskot and Hobza 2004; Macas et al. 2011). Multiple sex chromosomes, such as XY1Y2, were also reported in several plant species (Hizume et al. 1988; Howell et al. 2009; Mariotti et al. 2009; Navajas-Perez et al. 2009). Most of the plant species with sex chromosomes have not been sequenced. We have limited knowledge of the evolutionary histories of plant sex chromosomes because of scarce genomic resources and a lack of cytological and comparative studies of genetically related plant species with sex chromosomes.
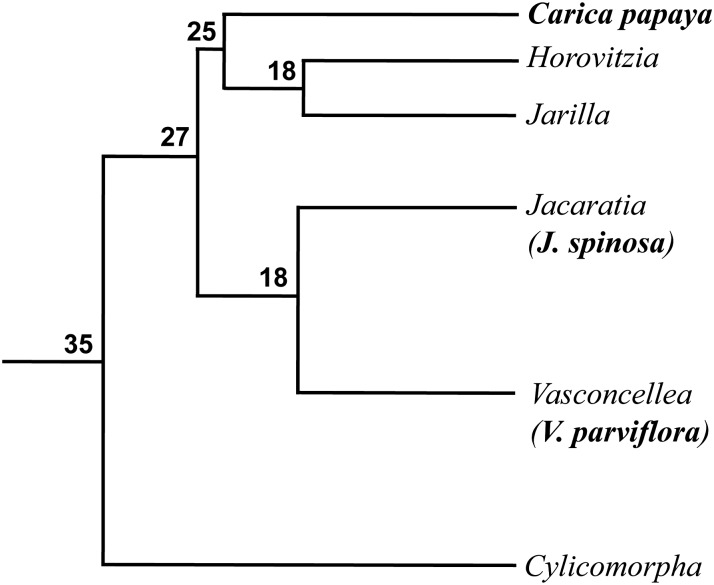
Most species in the Caricaceae family are dioecious. Therefore, dioecy likely represents the ancestral sexual state in this family (Charlesworth 2013). Papaya (Carica papaya) (2n = 2x = 18), an important fruit crop in Caricaceae, contains a pair of X/Y chromosomes that emerged only a few million years ago (MYA) (Yu et al. 2008; Wang et al. 2012). The young nature of papaya sex chromosomes was confirmed cytologically in which only a small portion (∼13%) of the X/Y chromosomes is cytologically differentiated (Zhang et al. 2008). Thus, papaya provides a model system to capture early molecular and cytological events during sex chromosome evolution. The Vasconcellea/Jacaratia clade in Caricaceae is closely related to papaya (Carvalho and Renner 2012) (Figure 1). We were intrigued to know whether the dioecious Vasconcellea/Jacaratia species contains sex chromosomes and whether such sex chromosomes are related to the papaya X/Y chromosome. We conducted fluorescence in situ hybridization (FISH) of a set of bacterial artificial chromosome (BAC) clones, all of which were previously mapped to the papaya X/Y chromosomes, in two Caricaceae species, Vasconcellea parviflora (2n = 2x = 18) and Jacaratia spinosa (2n = 2x = 18). We demonstrate that V. parviflora contains a pair of heteromorphic X/Y chromosomes that are homologous to the papaya X/Y chromosomes. By contrast, the same pair of chromosomes in J. spinosa is homomorphic. These results reveal a dynamic nature of sex chromosome evolution in the Caricaceae species.
Figure 1.
Phylogenetic relationships in family Caricaceae. Branch lengths are proportional to time. Numbers on nodes represent age in million years. This chronogram is adapted from Carvalho and Renner (2012). Species sampled in this study are in boldface type.
Materials and Methods
Materials
Plants of V. parviflora and J. spinosa were maintained at the Hawaii Agriculture Research Center as well as in the Texas AgriLife Research Center in Weslaco, Texas. Young male flower buds were collected and fixed in 3:1 (100% ethanol:glacial acetic acid) Carnoy’s solution and kept at −20° until use. Twenty-nine papaya BAC clones were previously selected and were either specific to the papaya sex-determining region (X or Y specific) or to the papaya sex chromosomes (Zhang et al. 2008; Wai et al. 2012). A telomeric DNA probe, pAtT4 from Arabidopsis thaliana (Richards and Ausubel 1988), was used to label the ends of V. parviflora pachytene chromosomes.
FISH
Chromosome preparation and FISH followed previously published protocols (Cheng et al. 2002; Iovene et al. 2008). BAC DNA was labeled with either biotin-16-dUTP or digoxigenin-11-dUTP (Roche Diagnostics, Indianapolis, IN), using a standard nick translation reaction. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in VectaShield antifade solution (Vector Laboratories, Burlingame, CA). The FISH images were processed with Meta Imaging Series 7.5 software. The final contrast of the images was processed using Adobe Photoshop CS3 software. Heterochromatin length was estimated based on DAPI staining pattern of the putative Y chromosome and expressed in percentage of the total chromosome length. The Y-specific heterochromatic domain was estimated as the difference in heterochromatin content between X and Y chromosomes and was expressed as the percentage of the total chromosome length. The position of a BAC clone or other landmarks along the chromosome was estimated and expressed as D/L × 100, where D = distance of the landmark from the end of the short arm, and L = total chromosome length. All measurements were made on digital photographs, using Meta Imaging Series 7.5 software.
Results
Identification of the X/Y chromosomes in V. parviflora
V. parviflora is a dioecious species with female and male individuals. Both male and female flowers are morphologically similar to those of papaya (Figure 2). Young flower buds were collected from male V. parviflora plants to prepare meiotic pachytene chromosomes. The pachytene karyotype of V. parviflora generally resembled the papaya karyotype (Zhang et al. 2010), containing nine metacentric and submetacentric chromosomes of similar sizes (Figure 3C). Heterochromatin, which is brightly stained by DAPI, was visible in the pericentromeric regions of all nine pachytene chromosomes (Figure 3C). Surprisingly, a large loop was consistently observed on one of the pachytene chromosomes (Figure 3A). The two homologous chromosomes were separated and unpaired within the loop. A large and a small heterochromatic domain were observed on only one of the two homologs in the loop (Figure 3B).
Figure 2.
Morphology of flowers from papaya and V. parviflora. (A) Papaya male flowers. (B) Papaya female flowers, which occur individually or in clusters. (C) V. parviflora male flowers. (D) V. parviflora female inflorescence, which usually contains >10 flowers.
Figure 3.
Pachytene chromosomes of V. parviflora. (A) A complete pachytene cell of V. parviflora. The green FISH signals were derived from an Arabidopsis telomeric DNA probe pAtT4. The red FISH signal (arrow) was derived from BAC 1, which is specific to papaya X/Y chromosomes. The loop on the X/Y chromosome pair is included in a white box. Bar, 10 μm. (B) The DAPI-stained chromosomes in A were converted into a black-and-white image to enhance the visualization of heterochromatin. The X (red) and Y (green) chromosomes within the loop are illustrated in the inset. A large Y-specific heterochromatin domain in the loop is illustrated in blue. (C) A pachytene karyotype developed from the same cell as in A. Individual chromosomes were digitally isolated, straightened, and arranged based on their length, except that the X/Y chromosome pair was arranged as the first. The arrow points to the FISH signal derived from BAC 1.
We next conducted FISH analysis on V. parviflora pachytene cells, using several BAC clones that were previously mapped to the papaya X/Y chromosomes. Most of these BACs were mapped to this loop-associated V. parviflora chromosome pair, suggesting that they are homologous to the papaya X/Y chromosomes (Figure 3C). Therefore, this pachytene chromosome pair is likely the sex chromosome pair in V. parviflora and the loop region represents the MSY. Significant accumulation of heterochromatin and possibly chromosomal rearrangements, such as inversions and/or duplications, within the loop may prevent pairing of the putative X and Y chromosomes in this region.
Distinct heterochromatin distribution patterns associated with the V. parviflora X/Y chromosomes
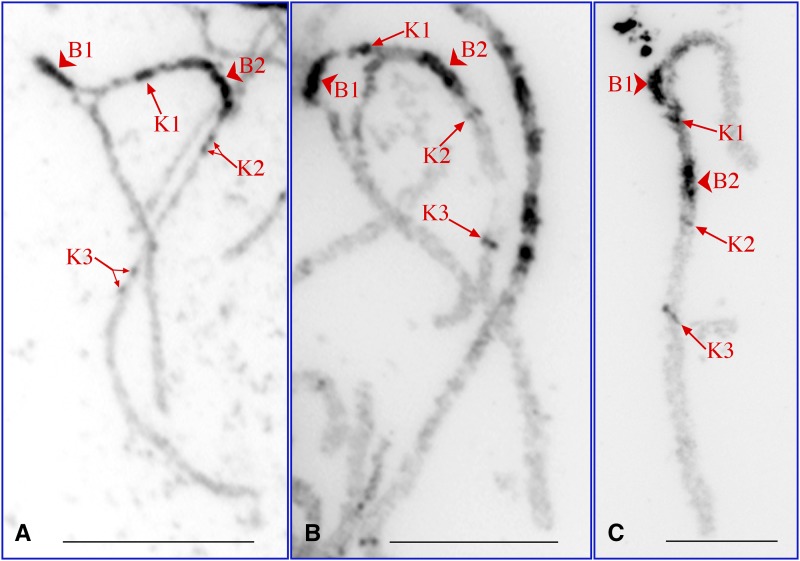
We prepared pachytene chromosomes at both early and late stages to further investigate chromatin differentiation between the X and Y chromosomes. Two large heterochromatin blocks (B), B1 and B2, and three small heterochromatic knobs (K), K1, K2, and K3, were observed on the X/Y chromosomes (Figure 4). These heterochromatic domains were best visualized on middle pachytene chromosomes (Figure 4B). B1 and K1 were observed only on one of the two homologs, most likely the Y chromosome. B1 was consistently located in the loop on both early and late pachytene chromosomes. K1 was much smaller than B1, but was detectable within the loop on most pachytene chromosomes. Heterochromatic domains were often divided into two or more separate subdomains on highly extended early pachytene chromosomes (Figure 4A).
Figure 4.
Heterochromatin distribution along the V. parviflora X/Y chromosomes. (A) An early pachytene chromosome. Two separated subdomains are visible from K1 and K2. The B1 region is self-supercoiled. (B) A middle pachytene chromosome. All five heterochromatin domains are clearly visible. B1 and K1 are located in the loop and are associated with only one of the two homologs. (C) A late pachytene chromosome. The chromosomes were stained by DAPI and were converted into black-and-white images. The five heterochromatic blocks (B1, B2) or knobs (K1, K2, K3) are indicated by red arrowheads and arrows. Bars, 10 μm.
The heterochromatin distribution pattern on V. parviflora X/Y chromosomes is similar to the pattern observed on papaya X/Y chromosomes. The papaya X/Y pachytene chromosome contains five knobs, including one large knob shared by X and Y and four small knobs specific to the papaya MSY (Zhang et al. 2008). The loop associated with the V. parviflora X/Y chromosomes is located at a similar position to that of the MSY on the papaya Y chromosome (Figure 5). The length of the loop accounted for 12% (11.7 ± 3.04, n = 15) of the V. parviflora Y chromosome. In comparison, the MSY of papaya accounts for 13% of the papaya Y chromosome (Zhang et al. 2008). However, more heterochromatin has accumulated in the V. parviflora MSY than in the papaya MSY. No loop was observed in the papaya MSY, although the Y chromosome, which contains 8.1 Mb DNA compared to 3.5 Mb for the corresponding X region (Wang et al. 2012), has to twist (zigzag) in pairing with the X within the MSY (Zhang et al. 2008). In comparison, the V. parviflora Y chromosome appeared to have accumulated much more DNA than the X chromosome in the loop. Self “pairing” or “supercoiling” of the Y chromosome was observed within the loop on early pachytene chromosomes (Figure 4A).
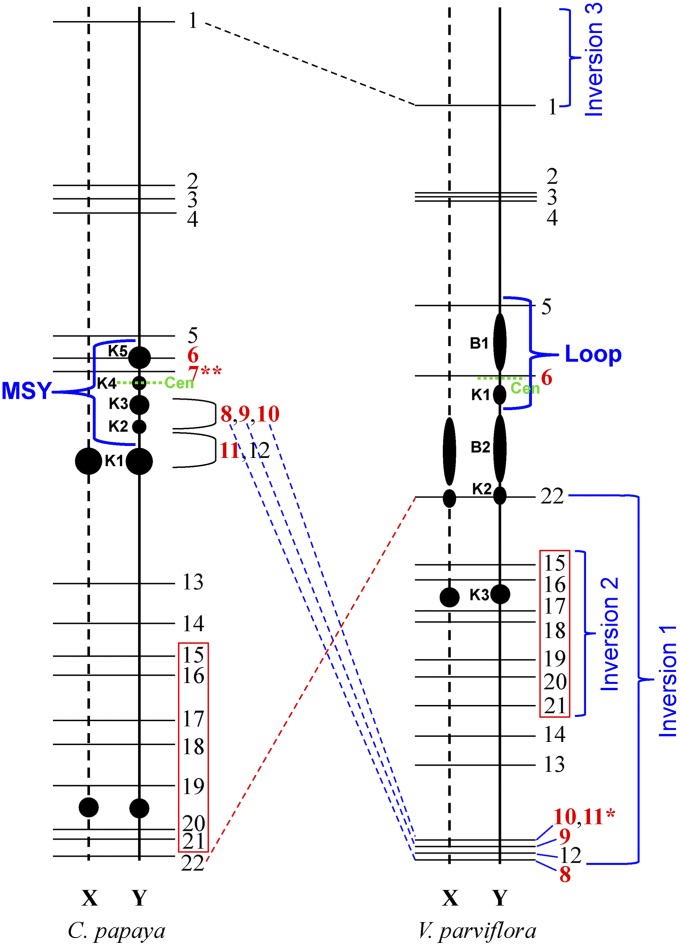
Figure 5.
Comparative mapping of the X/Y chromosomes in papaya (adapted from Zhang et al. 2010; Wai et al. 2012) and V. parviflora. BACs in red numbers in boldface type are specific to the papaya X chromosome. BAC 11* generated signals on an additional V. parviflora chromosome. BAC 7** was mapped to a different chromosome in V. parviflora. The green dashed lines mark the putative positions of the centromeres on the Y chromosomes. The position of the papaya MSY and the loop on V. parviflora Y chromosomes are marked. Three inversions are predicted based on the order and position of BAC clones in the two species. The different locations of BAC 1 in the two species are illustrated by a black dashed line. Blue and red dashed lines highlight the centromeric vs. telomeric positions of the four BACs in the two species. Note that the three inversions drawn along the V. parviflora Y chromosome likely emerged during the evolution of papaya.
The centromere of the papaya Y chromosome was mapped close to knob 4 within the MSY (Zhang et al. 2008) (Figure 5). The chromosomal domain between B1 and K1 in the V. parviflora MSY was consistently less stained and appeared morphologically to be the primary constriction. Based on this structure, as well as the synteny between papaya X/Y and V. parviflora X/Y chromosomes (see below), the centromere of the V. parviflora Y chromosome is predicted to be located between B1 and K1 within the loop (Figure 5).
Comparative FISH mapping of the X/Y chromosomes in papaya and V. parviflora
We selected a total of 29 BAC clones that were previously mapped to the papaya X/Y chromosomes (Zhang et al. 2008; Wai et al. 2010; Na et al. 2012), including 11 BACs specific to X, 1 BAC specific to Y, and 17 BACs shared by X and Y (Supporting Information, Table S1). These BACs were mapped to the homologous V. parviflora chromosomes by FISH to further examine the syntenic relationship of the sex chromosomes in the two species. Only 22 papaya BACs generated distinct FISH signals on V. parviflora chromosomes. These clones were labeled in numbers from 1 to 22 (Figure 5, Table S1).
Five BACs, including clones 8–11 that are located in the papaya MSY, were surprisingly mapped to the distal region on the long arm of V. parviflora X/Y chromosomes (Figure 5). By contrast, BAC 22, the most distal clone on the long arm of papaya X/Y chromosomes, was mapped to the middle of the V. parviflora X/Y chromosomes (Figure 5 and Figure 6C). Thus, an inversion (inversion 1 in Figure 5), which spans almost the entire long arm, occurred in one of the two species after divergence from a common ancestor. The reverse order of BACs 13 and 14 in the two species (Figure 5) also supports this inversion hypothesis. Interestingly, BACs 15–21 maintained the same order on the long arm in the two species (Figure 5 and Figure 6E). Thus, an independent and smaller inversion (inversion 2 in Figure 5) spanning BACs 15–21 occurred within the chromosomal domain spanned by inversion 1.
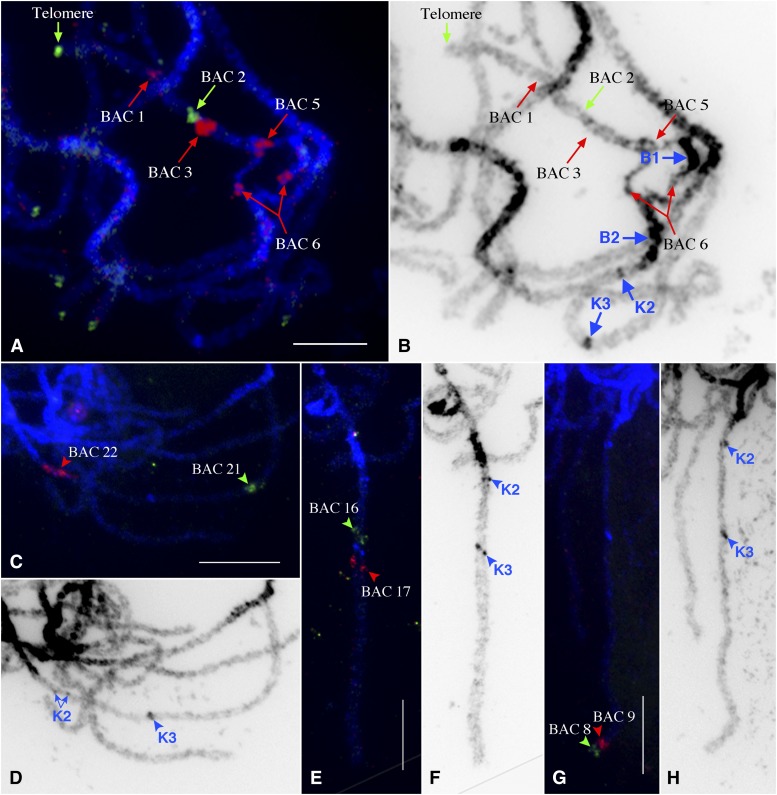
Figure 6.
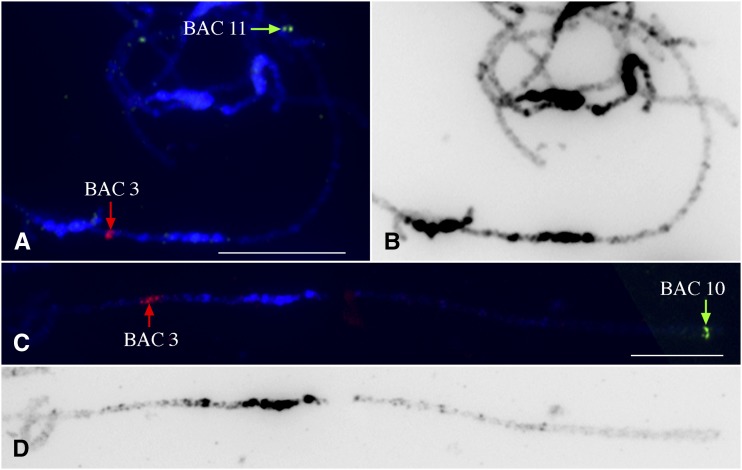
FISH mapping of papaya BACs on V. parviflora X/Y chromosomes. (A) FISH signals derived from five papaya BACs and a telomeric DNA probe on V. parviflora X/Y chromosomes. BAC 5 is located at the short arm boundary of the loop. BAC 6 is located in the middle of the loop. The signals from BAC 6 on the X and Y chromosomes are separated and are indicated by a double arrow. (B) Chromosomes in A were converted into a black-and-white image to enhance the heterochromatic features. (C) FISH signals derived from BACs 21 and 22. These two BACs are located at the distal end of papaya X/Y chromosomes (Figure 5). BAC 22, however, is located in the middle of the V. parviflora X/Y chromosomes. (D) Chromosomes in C were converted into a black-and-white image. (E) FISH signals derived from BACs 16 and 17, which span K3. (F) Chromosomes in E were converted into a black-and-white image. (G) FISH signals derived from BACs 8 and 9. These two BACs are located within the MSY of papaya X/Y chromosomes (Figure 5), but are located at the distal end of the long arm of V. parviflora X/Y chromosomes. (H) Chromosomes in G were converted into a black-and-white image. Unambiguously identified heterochromatic blocks and knobs are marked by blue arrows or arrowheads in B, D, F, and H. Bars, 10 μm.
BACs 1–6, which span the short arm of papaya X/Y chromosomes, maintained the same order on V. parviflora X/Y chromosomes (Figure 5 and Figure 6A). However, the distance between BAC 1 and the telomere was much shorter in papaya than in V. parviflora, suggesting another inversion in this region (inversion 3 in Figure 5).
Comparative FISH mapping of the MSYs in papaya and V. parviflora
Although the two MSYs shared a similar chromosomal location and size, they differed in structure and DNA composition. The chromosomal domain spanned by BACs 8–11 represented a significant part of the papaya MSY. This region, however, was located at the distal end of the long arm of V. parviflora X/Y chromosomes (Figure 5). In addition, BAC 7 was not mapped to the V. parviflora MSY, but to a different chromosome in V. parviflora. We also examined the location of the 5S ribosomal RNA genes (rDNA) in V. parviflora. A single 5S rDNA locus was present on a pair of V. parviflora autosomes. No 5S rDNA sequences were detected in the V. parviflora MSY (Figure S1). By contrast, massive amounts of 5S rDNA-related sequences were amplified in the papaya MSY (Zhang et al. 2010).
Based on the position of the Y chromosome centromere within the MSY, the majority of the papaya MSY is located on the long arm of the Y chromosome (Zhang et al. 2008). In contrast, the heterochromatic domain B1 represents more than half of the V. parviflora MSY (Figure 4). This region, spanned by BACs 5 and 6, was significantly expanded in V. parviflora compared to the same region in papaya (Figure 5). Since the centromere of the V. parviflora Y chromosome is located between K1 and B1, the majority of the V. parviflora MSY is located on the short arm of the Y chromosome (Figure 5). Thus, the papaya MSY has expanded toward the long arm of the Y chromosome, whereas the V. parviflora MSY has expanded toward the short arm of the Y chromosome.
J. spinosa chromosomes homologous to papaya X/Y chromosomes are homomorphic
Jacaratia is one of six genera in Caricaceae. Phylogenetic analysis revealed that Jacaratia and Vasconcellea species are equally distant from papaya (Carvalho and Renner 2012) (Figure 1). We were interested whether Jacaratia species also shared the same sex chromosomes with papaya and V. parviflora. We chose one dioecious species, J. spinosa (2n = 2x = 18), for comparative FISH mapping.
We conducted FISH analysis, using nine papaya BAC clones (BACs 3 and 5–12) (Table S2) on J. spinosa chromosomes. The chromosomal positions of all nine BACs in J. spinosa were similar to those observed on V. parviflora X/Y chromosomes, including the distal locations of BACs 8–12 (Figure 7 and Table S2). Therefore, the three inversions associated with this chromosome in Caricaceae species are not specific to V. parviflora and may have emerged during the evolution of the papaya genome.
Figure 7.
FISH mapping in J. spinosa. (A) FISH mapping of BACs 3 and 11. Chromosomal locations of these two clones on the J. spinosa chromosome are similar to those on V. parviflora X/Y chromosomes. (B) Chromosomes in A were converted into a black-and-white image. The J. spinosa chromosomes are homomorphic and show a complete chromosome pairing. (C) FISH mapping of BACs 3 and 10. Chromosomal locations of these two clones on the J. spinosa chromosome are similar to those on V. parviflora X/Y chromosomes. (D) Chromosomes in C were converted into a black-and-white image. The J. spinosa chromosomes are homomorphic and show a complete chromosome pairing.
Surprisingly, the J. spinosa chromosome pair that is homologous to the papaya/V. parviflora X/Y chromosomes showed perfect pairing at the pachytene stage without any loop. No chromatin differentiation between the two homologs was observed on this pachytene chromosome (Figure 7, B and D). We did not observe any heteromorphic pachytene chromosome in J. spinosa. However, it is not conclusive regarding the absence of heteromorphic sex chromosomes in this species because we were able to collect and analyze only few meiotic cells at the pachytene stage.
Discussion
Sex chromosomes emerged multiple times independently in several eukaryotic lineages, including fishes, reptiles, and plants (Graves 2008; Ming et al. 2011; Bachtrog 2013). For example, different sex chromosomes evolved independently among several closely related fish species (Woram et al. 2003; Takehana et al. 2007, 2008). Sex chromosomes have been best studied in the medaka fish Oryzias latipes (Matsuda et al. 2002, 2007). Interestingly, the sex-determining gene in O. latipes, DMY, was not detected in a sister species O. luzonensis. Instead, a novel sex-determining gene located on a different chromosome may have arisen in O. luzonensis and replaced DMY (Tanaka et al. 2007). Similarly, S. latifolia is a well-studied model plant with X/Y chromosomes (Vyskot and Hobza 2004). S. colpophylla, a species closely related to S. latifolia, contains sex chromosomes evolved from a different pair of autosomes (Mrackova et al. 2008).
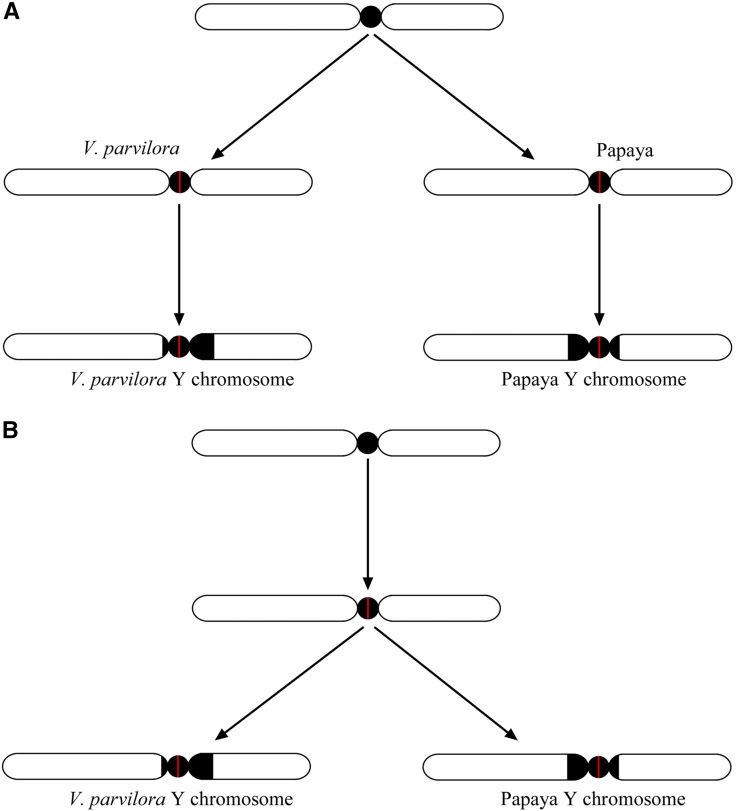
In this study, we demonstrated that V. parviflora contains a pair of X/Y chromosomes that are homologous to those of papaya. An interesting question regarding the results of the current study is whether the X/Y chromosomes in papaya and V. parviflora evolved independently from the same pair of autosomes or from the same pair of ancestral X/Y chromosomes. Since the papaya X/Y chromosomes are estimated to have emerged approximately 7 MYA (Yu et al. 2008; Wang et al. 2012) and the Vasconcellea/Jacaratia species are estimated to have diverged from the papaya clade ∼27 MYA (Carvalho and Renner 2012), the sex chromosomes in papaya and V. parviflora may have originated independently from the same pair of autosomes (Figure 8A). However, we must be cautious in drawing such a conclusion because the age estimation of the papaya sex chromosomes was based on a limited amount of DNA sequences and on the nucleotide substitution rates of different plant species (Wang et al. 2012). Additional sequence evidence and calculations will be required to support the relative young age of the papaya sex chromosomes vs. the divergence time of the Caricaceae species.
Figure 8.
Models of Y chromosome evolution in papaya and V. parviflora. (A) Independent evolution model. A sex-determining gene (red bar) located in the centromeric region emerged independently in papaya and V. parviflora, respectively. The two ancestral Y chromosomes then evolved into the current Y chromosomes in the two species. (B) Monophyletic evolution model. The two current Y chromosomes in papaya and V. parviflora evolved from the same ancestral Y chromosome.
An alternative interpretation is that the key sex-determining gene(s) emerged before the divergence of papaya and V. parviflora (Figure 8B). The similar physical sizes of the two MSYs suggest that the X/Y chromosomes in papaya and V. parviflora may have emerged at a similar time. The MSYs in the two species are located at a similar position on the two Y chromosomes (Figure 5). Remarkably, comparative FISH mapping revealed that the two MSYs shared only a small chromosomal domain that is marked by BAC 6 and is close to the centromere of the two Y chromosomes (Figure 5). Thus, the sex-determining gene(s) in papaya and V. parviflora are likely located either within the centromere or closely flanking the centromere of the Y chromosomes. Active genes were reported in the centromeres of plant chromosomes, including rice (Nagaki et al. 2004; Yan et al. 2006) and potato (Gong et al. 2012). In addition, chromosomal crossings over are completely suppressed within centromeres as well as regions immediately flanking the centromeres (Yan et al. 2005, 2008). Thus, the location of the newly emerged sex-determining gene(s) in the centromeric region is favorable for the survival of these genes during evolution.
If the starting position of the two MSYs is around the centromere, then the papaya MSY has expanded toward the long arm of the chromosome and the V. parviflora MSY toward the short arm (Figure 5). The differential expansions of the two MSYs are also vindicated by several cytogenetic mapping results:
The V. parviflora MSY is more heterochromatic than the papaya MSY. The V. parviflora MSY is consistently associated with an unpaired chromosomal loop, whereas the X and Y chromosomes within papaya MSY can pair, although in an irregular fashion (Zhang et al. 2008).
The papaya MSY has accumulated massive amounts of repetitive DNA sequences derived from 5S ribosomal RNA genes (Zhang et al. 2010). By contrast, we did not detect any 5S rDNA-related DNA sequences in V. parviflora MSY (Figure S1).
Several BACs showed different hybridization patterns in the two MSYs. BAC 7 is associated with papaya MSY, but it hybridizes to a different chromosome in V. parviflora. BAC 6 is specific to X in papaya, but it hybridizes to both X and Y in V. parviflora.
J. spinosa contains a pair of chromosomes that share a perfect synteny with the V. parviflora X/Y chromosomes (Table S2). Interestingly, no loop or chromatin differentiation was observed on this pair of J. spinosa chromosomes at the pachytene stage. These results can be interpreted by two hypotheses: (1) Sex determination in J. spinosa is associated with the same pair of chromosomes as papaya and V. parviflora, but the primitive X/Y chromosomes in J. spinosa have not differentiated cytologically and appear to be homomorphic, and (2) a different pair of chromosomes is associated with sex determination in J. spinosa. Thus, J. spinosa provides an excellent model for future study of sex chromosome evolution in Caricaceae species.
Acknowledgments
We thank Fernanda Carvalho for help on the development of Figure 1 and Li He for development of Figure 3C in the manuscript. We are grateful to Francis Zee, Jim Carr, and other members of the Zee laboratory for help in the collection of meiotic samples of Vasconcellea/Jacaratia species. This work was supported by National Science Foundation Plant Genome Research Program awards DBI0553417 and DBI0922545.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.173021/-/DC1.
Communicating editor: D. A. Barbash
Literature Cited
- Bachtrog D., 2013. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 14: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellott D. W., Skaletsky H., Pyntikova T., Mardis E. R., Graves T., et al. , 2010. Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition. Nature 466: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho F. A., Renner S. S., 2012. A dated phylogeny of the papaya family (Caricaceae) reveals the crop’s closest relatives and the family’s biogeographic history. Mol. Phylogenet. Evol. 65: 46–53. [DOI] [PubMed] [Google Scholar]
- Charlesworth D., 2013. Plant sex chromosome evolution. J. Exp. Bot. 64: 405–420. [DOI] [PubMed] [Google Scholar]
- Cheng Z. K., Buell C. R., Wing R. A., Jiang J. M., 2002. Resolution of fluorescence in-situ hybridization mapping on rice mitotic prometaphase chromosomes, meiotic pachytene chromosomes and extended DNA fibers. Chromosome Res. 10: 379–387. [DOI] [PubMed] [Google Scholar]
- Cortez D., Marin R., Toledo-Flores D., Froidevaux L., Liechti A., et al. , 2014. Origins and functional evolution of Y chromosomes across mammals. Nature 508: 488–493. [DOI] [PubMed] [Google Scholar]
- Gong Z. Y., Wu Y. F., Koblizkova A., Torres G. A., Wang K., et al. , 2012. Repeatless and repeat-based centromeres in potato: implications for centromere evolution. Plant Cell 24: 3559–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. A. M., 2008. Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annu. Rev. Genet. 42: 565–586. [DOI] [PubMed] [Google Scholar]
- Hizume M., Shiraishi H., Tanaka A., 1988. A cytological study of Podocarpus macrophyllus with special reference to sex chromosomes. Jap. J. Genet. 63: 413–423. [Google Scholar]
- Howell E. C., Armstrong S. J., Filatov D. A., 2009. Evolution of neo-sex chromosomes in Silene diclinis. Genetics 182: 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovene M., Wielgus S. M., Simon P. W., Buell C. R., Jiang J. M., 2008. Chromatin structure and physical mapping of chromosome 6 of potato and comparative analyses with tomato. Genetics 180: 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kumari R., Sharma V., 2014. Genetics of dioecy and causal sex chromosomes in plants. J. Genet. 93: 241–277. [DOI] [PubMed] [Google Scholar]
- Macas J., Kejnovsky E., Neumann P., Novak P., Koblizkova A., et al. , 2011. Next generation sequencing-based analysis of repetitive DNA in the model dioceous plant Silene latifolia. PLoS ONE 6: e27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti B., Manzano S., Kejnovsky E., Vyskot B., Jamilena M., 2009. Accumulation of Y-specific satellite DNAs during the evolution of Rumex acetosa sex chromosomes. Mol. Genet. Genomics 281: 249–259. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Nagahama Y., Shinomiya A., Sato T., Matsuda C., et al. , 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417: 559–563. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Shinomiya A., Kinoshita M., Suzuki A., Kobayashi T., et al. , 2007. DMY gene induces male development in genetically female (XX) medaka fish. Proc. Natl. Acad. Sci. USA 104: 3865–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming R., Bendahmane A., Renner S. S., 2011. Sex chromosomes in land plants. Annu. Rev. Plant Biol. 62: 485–514. [DOI] [PubMed] [Google Scholar]
- Mrackova M., Nicolas M., Hobza R., Negrutiu I., Moneger F., et al. , 2008. Independent origin of sex chromosomes in two species of the genus Silene. Genetics 179: 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J. K., Wang J. P., Murray J. E., Gschwend A. R., Zhang W. L., et al. , 2012. Construction of physical maps for the sex-specific regions of papaya sex chromosomes. BMC Genomics 13: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K., Cheng Z. K., Ouyang S., Talbert P. B., Kim M., et al. , 2004. Sequencing of a rice centromere uncovers active genes. Nat. Genet. 36: 138–145. [DOI] [PubMed] [Google Scholar]
- Navajas-Perez R., Schwarzacher T., Rejon M. R., Garrido-Ramos M. A., 2009. Molecular cytogenetic characterization of Rumex papillaris, a dioecious plant with an XX/XY1Y2 sex chromosome system. Genetica 135: 87–93. [DOI] [PubMed] [Google Scholar]
- Richards E. J., Ausubel F. M., 1988. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell 53: 127–136. [DOI] [PubMed] [Google Scholar]
- Sousa A., Fuchs J., Renner S. S., 2013. Molecular cytogenetics (FISH, GISH) of Coccinia grandis: a ca. 3 myr-old species of Cucurbitaceae with the largest Y/autosome divergence in flowering plants. Cytogenet. Genome Res. 139: 107–118. [DOI] [PubMed] [Google Scholar]
- Takehana Y., Demiyah D., Naruse K., Hamaguchi S., Sakaizumi M., 2007. Evolution of different Y chromosomes in two medaka species, Oryzias dancena and O. latipes. Genetics 175: 1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana Y., Hamaguchi S., Sakaizumi M., 2008. Different origins of ZZ/ZW sex chromosomes in closely related medaka fishes, Oryzias javanicus and O. hubbsi. Chromosome Res. 16: 801–811. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Takehana Y., Naruse K., Hamaguchi S., Sakaizumi M., 2007. Evidence for different origins of sex chromosomes in closely related Oryzias fishes: substitution of the master sex-determining gene. Genetics 177: 2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgmann-Rauber A., Jamsari A., Kinney M. S., Pires J. C., Jung C., 2007. Genetic and physical maps around the sex-determining M-locus of the dioecious plant asparagus. Mol. Genet. Genomics 278: 221–234. [DOI] [PubMed] [Google Scholar]
- Vyskot B., Hobza R., 2004. Gender in plants: sex chromosomes are emerging from the fog. Trends Genet. 20: 432–438. [DOI] [PubMed] [Google Scholar]
- Wai C. M., Yu Q. Y., Moore P. H., Paull R. E., Ming R., 2010. Development of chromosome-specific cytogenetic markers and merging of linkage fragments in papaya. Tropical Plant Biol. 3: 171–181. [Google Scholar]
- Wai C. M., Moore P. H., Paull R. E., Ming R., Yu Q. Y., 2012. An integrated cytogenetic and physical map reveals unevenly distributed recombination spots along the papaya sex chromosomes. Chromosome Res. 20: 753–767. [DOI] [PubMed] [Google Scholar]
- Wang J. P., Na J. K., Yu Q. Y., Gschwend A. R., Han J., et al. , 2012. Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc. Natl. Acad. Sci. USA 109: 13710–13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woram R. A., Gharbi K., Sakamoto T., Hoyheim B., Holm L. E., et al. , 2003. Comparative genome analysis of the primary sex-determining locus in salmonid fishes. Genome Res. 13: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Oda Y., Haseda A., Fujito S., Mikami T., et al. , 2014. Molecular evidence that the genes for dioecism and monoecism in Spinacia oleracea L. are located at different loci in a chromosomal region. Heredity 112: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato K. T., Ishizaki K., Fujisawa M., Okada S., Nakayama S., et al. , 2007. Gene organization of the liverwort Y chromosome reveals distinct sex chromosome evolution in a haploid system. Proc. Natl. Acad. Sci. USA 104: 6472–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Jin W. W., Nagaki K., Tian S., Ouyang S., et al. , 2005. Transcription and histone modifications in the recombination-free region spanning a rice centromere. Plant Cell 17: 3227–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Ito H., Nobuta K., Ouyang S., Jin W. W., et al. , 2006. Genomic and genetic characterization of rice Cen3 reveals extensive transcription and evolutionary implications of a complex centromere. Plant Cell 18: 2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H., Talbert P. B., Lee H. R., Jett J., Henikoff S., et al. , 2008. Intergenic locations of rice centromeric chromatin. PLoS Biol. 6: 2563–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T., DiFazio S. P., Gunter L. E., Zhang X., Sewell M. M., et al. , 2008. Genome structure and emerging evidence of an incipient sex chromosome in Populus. Genome Res. 18: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q. Y., Hou S., Feltus F. A., Jones M. R., Murray J. E., et al. , 2008. Low X/Y divergence in four pairs of papaya sex-linked genes. Plant J. 53: 124–132. [DOI] [PubMed] [Google Scholar]
- Zhang W. L., Wang X. U., Yu Q. Y., Ming R., Jiang J. M., 2008. DNA methylation and heterochromatinization in the male-specific region of the primitive Y chromosome of papaya. Genome Res. 18: 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. L., Wai C. M., Ming R., Yu Q. Y., Jiang J. M., 2010. Integration of genetic and cytological maps and development of a pachytene chromosome-based karyotype in papaya. Tropical Plant Biol. 3: 166–170. [Google Scholar]