Abstract
Background and objectives
AKI in critically ill patients is usually part of multiorgan failure. However, nonrenal organ failure may not always precede AKI and patients without evidence of these organ failures may not be at low risk for AKI. This study examined the risk and outcomes associated with AKI in critically ill patients with and without cardiovascular or respiratory organ failures at presentation to the intensive care unit (ICU).
Design, setting, participants, & measurements
A large, academic medical center database, with records from July 2000 through October 2008, was used and the authors identified a low-risk cohort as patients without cardiovascular and respiratory organ failures defined as not receiving vasopressor support or mechanical ventilation within the first 24 hours of ICU admission. AKI was defined using Kidney Disease Improving Global Outcomes criteria. The primary end points were moderate to severe AKI (stages 2–3) and risk-adjusted hospital mortality.
Results
Of 40,152 critically ill patients, 44.9% received neither vasopressors nor mechanical ventilation on ICU day 1. Stages 2–3 AKI occurred less frequently in the low-risk patients versus high-risk patients within 24 hours (14.3% versus 29.1%) and within 1 week (25.7% versus 51.7%) of ICU admission. Patients developing AKI in both risk groups had higher risk of death before hospital discharge. However, the adjusted odds of hospital mortality were greater (odds ratio, 2.99; 95% confidence interval, 2.62 to 3.41) when AKI occurred in low-risk patients compared with those with respiratory or cardiovascular failures (odds ratio, 1.19; 95% confidence interval, 1.09 to 1.3); interaction P<0.001.
Conclusions
Patients admitted to ICU without respiratory or cardiovascular failure have a substantial likelihood of developing AKI. Although survival for low-risk patients is better than for high-risk patients, the relative increase in mortality associated with AKI is actually greater for low-risk patients. Strategies aimed at preventing AKI should not exclude ICU patients without cardiovascular or respiratory organ failures.
Keywords: mortality, outcomes, kidney disease
Introduction
We previously reported that one third of patients hospitalized for community-acquired pneumonia develop AKI and have significantly lower 1-year survival compared with those that do not (1). Furthermore, we found that AKI was common even in patients outside the intensive care unit (ICU) or with nonsevere pneumonia (1). Indeed, although critical illness is a major predisposing factor for AKI, AKI may manifest before other organ failures. Patients without other organ failures, especially those without respiratory or cardiovascular dysfunction, are often less critically ill and therefore assumed to be less likely to develop AKI. Such patients are judged to be at lower risk for developing AKI and are less likely to receive recommended interventions for high-risk patients such as avoidance of unnecessary nephrotoxic drugs and radiocontrast, close monitoring of serum creatinine and urine output and assessment of fluid status (2). The National Health Service in the United Kingdom has systematically assessed the care and outcomes for patients with AKI, and found that inadequate risk-assessment is common and is associated with delays in treatment and investigators even judged some cases to have been avoidable altogether (3).
However, the consequences of AKI in lower risk ICU patients are not well understood and may not be as significant as in high-risk patients. Patients with sepsis and single-organ failure have far better outcomes compared with patients with multiple organ dysfunction (4). Therefore if the effect of AKI on lower risk patients is relatively low, quality improvement efforts might be better allocated to sicker patients. Because cardiovascular and respiratory organ failures are common in the context of systemic illnesses that also cause AKI (e.g., sepsis, polytrauma), we sought to examine the risk of AKI and the outcomes associated with AKI in patients with and without these organ failures. We also compared susceptibilities and exposures between patients in low- and high-risk categories.
Materials and Methods
Source Population
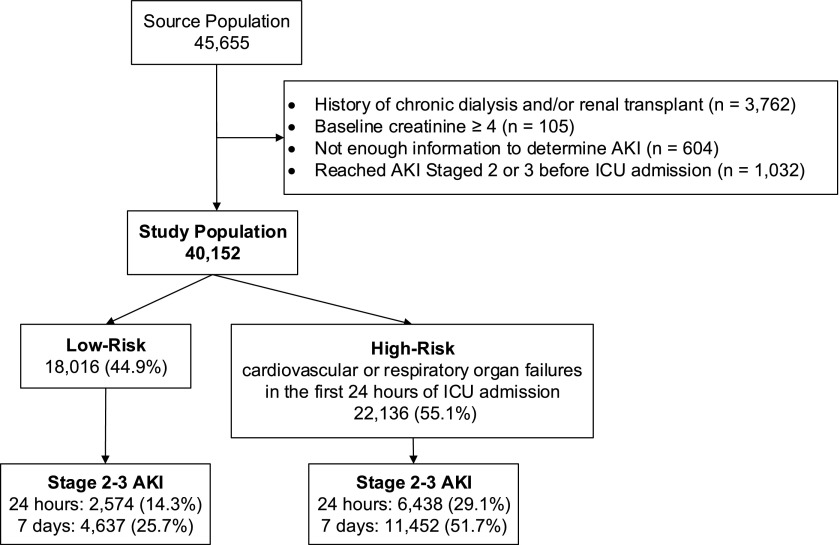
The High-Density Intensive Care (HiDenIC-8) database includes data on a source population of 45,655 adult patients admitted to one of eight ICUs (i.e., medical, cardiac, transplant, surgical, neurologic, and trauma) within a single academic medical center (University of Pittsburgh Medical Center [UPMC], Pittsburgh, PA) during an 8-year period (July 2000 through October 2008). For this study, we applied the following exclusion criteria: (1) hemodialysis or renal transplant before hospitalization (n=3762), (2) known baseline creatinine ≥4 (n=105), (3) insufficient information to determine AKI (n=604), and (4) stage 2 or stage 3 AKI before being admitted to ICU (n=1032). The remaining 40,152 patients comprised the study cohort (Figure 1).
Figure 1.
Study population selection. ICU, intensive care unit.
Data Collection
After obtaining institutional review board approval, we deidentified data using an honest broker. We obtained data from several computerized databases. Data on all patients admitted to an ICU at UPMC from 2000 to 2008 are contained in the HiDenIC-8 database, which merges data from several sources (5) containing demographics, diagnosis, billing codes, laboratory results, surgical procedures, and various text elements including reports, clinical notes, and discharge summaries. These data were combined with data from the Eclypsis database, the backbone of the inpatient electronic health record, which includes ICU patients’ physiology information including BP, respiration rate, temperature, heart rate, medications, fluids, mechanical ventilation, feeding, oxygen, details of RRT, and transfusions. The honest broker also obtained information from the US Renal Data System and the National Death Index and merged these results with the HiDenIC-8 database before deidentification.
Study Cohorts
We stratified patients into two cohorts: those with cardiovascular or respiratory organ failures in the first 24 hours of ICU admission (high risk) and those without (low risk). We defined cardiovascular and respiratory organ failures as receiving vasopressor support or mechanical ventilation. These levels of organ dysfunction correspond to a Sepsis-Related Organ Failure Assessment score of ≥2 for cardiovascular and ≥3 for respiratory (4). We further subdivided the low-risk patients that developed AKI into those that developed AKI after the start of mechanical ventilation or vasopressor support and those that developed AKI before or without these other organ failures. Comorbid conditions were determined by International Classification of Diseases, Ninth Revision (ICD-9) codes. We designated patients as surgical or medical based on the diagnosis-related group at hospital admission. The acute physiology score (APS-III) was computed from electronic abstraction of all physiologic variables comprising the Acute Physiologic and Chronic Health Evaluation (APACHE) III score as described (6). Because sepsis is underreported with ICD-9 codes, we defined “suspected sepsis” as the ordering of blood cultures and antibiotics within 24 hours of each other, as defined previously (7). To quantify the severity of hypotension, we calculated a hypotensive index that integrates the duration and depth of systolic BP<90 in the first 24 hours (Hi24) (area under the curve) after ICU admission.
Baseline, admission, and reference serum creatinine were determined as previously described (8,9). We defined baseline creatinine as the lowest value of (1) the most recent prehospital creatinine value up to 1 year before the index hospital admission and (2) creatinine recorded in the first 24 hours of hospital admission. Admission creatinine was the creatinine recorded in the first 24 hours of hospital admission. Reference creatinine was the baseline creatinine when available, otherwise the lowest between (1) admission creatinine, (2) creatinine in the first 24 hours of ICU, and (3) (for patients without history of CKD) creatinine derived from the Modification of Diet in Renal Disease equation using a GFR of 75 ml/min per 1.73 m2. In order to classify drugs as nephrotoxins, four drug information sources were reviewed. A drug was considered a “known” nephrotoxin if at least three of the drug information sources listed the drug as a nephrotoxin (10–13).
AKI Classification
Patients were classified according to the maximum Kidney Disease Improving Global Outcomes (KDIGO) criteria (2) met in the first 7 days after ICU admission. Any patient meeting the criteria for moderate to severe AKI (stages 2–3), either based on serum creatinine or on urine output, was considered to have AKI. Among those who required acute dialysis, only patients who reached stage 2 creatinine or urine output criteria before dialysis initiation were classified as having moderate to severe AKI.
Susceptibilities and Exposures
We reviewed the literature to identify risk factors for AKI and a recent meta-analysis of 31 observational studies in critically ill patients was used as a source of data on susceptibilities and exposures (14). We considered the following susceptibilities: age, sex, race, body mass index, comorbid conditions (i.e., diabetes, cardiac disease, chronic renal disease, hypertension), and eGFR; and exposures within 24 hours of ICU admission, including admission type (medical versus surgical), APS-III score, hypotension, suspected sepsis, and nephrotoxic drug use.
Outcome Assessment
Patient outcomes included ICU length of stay (LOS), hospital LOS, hospital mortality, and mortality at 30, 90, and 365 days from ICU admission.
Statistical Analyses
Statistical analyses were performed using STATA software (version SE 11.2), with statistical significance set at P value <0.05. Graphs were created using the R package ‘survival’ (version 2.37–7). Categorical variables were summarized as n (percentage) and continuous variables were summarized as mean (SD) if normally distributed and using the median±interquartile range if skewed. To determine whether the risk factors associated with AKI were the same between the high-risk and low-risk groups, we conducted multivariable logistic regression for each group. All variables were retained in the model regardless of significance level. To determine whether there was a difference in the magnitude of association between AKI and hospital mortality between the low- and high-risk groups, we conducted multivariable logistic regression and adjusted for several risk factors. We used Cox proportional hazards model to graphically describe the age-adjusted survival at 1 year after ICU admission by (1) no AKI/AKI classification and (2) KDIGO stage.
Results
Baseline Characteristics
Of the 40,152 patients meeting the inclusion criteria, 44.9% received neither mechanical ventilation nor vasopressor support within 24 hours of ICU admission (low-risk group). Fifteen percent of low-risk patients ultimately received mechanical ventilation or vasopressor support (after day 1). Baseline characteristics for low-risk and high-risk groups are shown in Table 1. Female sex, diabetes, heart failure, history of chronic renal disease, history of hypertension, and medical admission were more common in the low-risk group (P≤0.001). However, absolute differences were very small except medical admission. Severity of hypotension, sepsis, and RRT use were significantly greater in the high-risk patients (P<0.001). There was no difference in reference eGFR between groups (P=0.11).
Table 1.
Baseline characteristics for low-risk and high-risk groups
| Characteristica | Low Risk (n=18,016) | High Risk (n=22,136) | All (n=40,152) | P Valueb |
|---|---|---|---|---|
| Age, yr | <0.001 | |||
| 18–44 | 3667 (20.4) | 4318 (19.5) | 7985 (19.9) | |
| 45–64 | 6638 (36.9) | 8427 (38.1) | 15,065 (37.5) | |
| 65–74 | 3289 (18.3) | 4550 (20.6) | 7839 (19.5) | |
| ≥75 | 4417 (24.5) | 4830 (21.8) | 9247 (23) | |
| Men | 10,034 (55.7) | 12,883 (58.2) | 22,917 (57.1) | <0.001 |
| Race | <0.001 | |||
| White | 14,166 (78.6) | 17,457 (78.9) | 31,623 (78.8) | |
| Black | 1466 (8.1) | 1552 (7) | 3018 (7.5) | |
| Other | 2384 (13.2) | 3127 (14.1) | 5511 (13.7) | |
| BMI, kg/m2 (n=33,371) | 26.8 (23.3–31.1) | 26.6 (23.3–31.1) | 26.7 (23.3–31) | 0.22 |
| Comorbid condition | ||||
| Diabetes | 1217 (6.8) | 1272 (5.7) | 2489 (6.2) | <0.001 |
| Cardiac disease | 1543 (8.6) | 1986 (9) | 3529 (8.8) | 0.16 |
| Chronic renal disease | 317 (1.8) | 296 (1.3) | 613 (1.5) | 0.001 |
| History of hypertension | 5924 (32.9) | 6735 (30.4) | 12,659 (31.5) | <0.001 |
| Multiple comorbidity | 5669 (31.5) | 7204 (32.5) | 12,873 (32.1) | 0.02 |
| Surgical admission (n=37,496) | 8286 (49.5) | 13,453 (64.8) | 21,739 (58) | <0.001 |
| Creatinine, mg/dl | ||||
| Known baseline (n=15,622) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 0.18 |
| Hospital admission (n=37,272) | 0.9 (0.7–1.2) | 1 (0.8–1.3) | 0.9 (0.8–1.3) | <0.001 |
| Reference | 0.81 (0.7–1) | 0.9 (0.7–1) | 0.86 (0.7–1) | <0.001 |
| Reference eGFR, ml/min per 1.73 m2 | 0.11 | |||
| <30 | 344 (1.9) | 415 (1.9) | 759 (1.9) | |
| 30–60 | 1484 (8.2) | 1952 (8.8) | 3436 (8.6) | |
| >60 | 16,182 (89.9) | 19,756 (89.3) | 35,938 (89.5) | |
| APS-III score (n=39,997)c | 40 (29–53) | 67 (48–89) | 53 (36–75) | <0.001 |
| Severity of hypotensionc,d | 0 (0–0) | 0 (0–6.5) | 0 (0–2.1) | <0.001 |
| Vasopressor usec | n/a | 8560 (38.7) | 8560 (21.3) | n/a |
| Mechanical ventilationc | n/a | 20,401 (92.2) | 20,401 (50.8) | n/a |
| Suspected sepsisc | 1097 (6.1) | 3077 (13.9) | 4174 (10.4) | <0.001 |
| Nephrotoxic drugs | ||||
| ACEI/ARB | 2116 (11.7) | 950 (4.3) | 3066 (7.6) | <0.001 |
| Vancomycin | 1200 (6.7) | 3220 (14.5) | 4420 (11) | <0.001 |
| Aminoglycoside | 264 (1.5) | 476 (2.2) | 740 (1.8) | <0.001 |
| Other antibiotic | 836 (4.6) | 980 (4.4) | 1816 (4.5) | 0.31 |
| Calcineurin inhibitor | 437 (2.4) | 1737 (7.8) | 2174 (5.4) | <0.001 |
| Nonsteroidal | 4452 (24.7) | 3545 (16) | 7997 (19.9) | <0.001 |
| Diuretics | 2905 (16.1) | 3742 (16.9) | 6647 (16.6) | 0.04 |
| Other nephrotoxic drugs | 531 (2.9) | 1882 (8.5) | 2413 (6) | <0.001 |
| Admitting ICU service | ||||
| Medical | 2937 (16.3) | 2893 (13.1) | 5830 (14.5) | <0.001 |
| General surgical | 2874 (16) | 3380 (15.3) | 6254 (15.6) | 0.06 |
| Trauma | 697 (3.9) | 1565 (7.1) | 2262 (5.6) | <0.001 |
| Neurosurgical | 3404 (18.9) | 2546 (11.5) | 5950 (14.8) | <0.001 |
| Neurology | 742 (4.1) | 908 (4.1) | 1650 (4.1) | >0.99 |
| Cardiac | 3680 (20.4) | 2432 (11) | 6112 (15.2) | <0.001 |
| Cardiothoracic surgery | 1245 (6.9) | 4687 (21.2) | 5932 (14.8) | <0.001 |
| Liver transplant | 2437 (13.5) | 3725 (16.8) | 6162 (15.3) | <0.001 |
Data are presented as n (%) or median (Q1–Q3) unless otherwise indicated. BMI, body mass index; APS-III, acute physiology score; n/a, not applicable; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICU, intensive care unit.
Missing values for age (16 patients) and sex (2 patients); otherwise, the n for each variable is provided in parentheses.
P value for the comparison of low-risk and high-risk groups.
Measured within 24 hours after ICU admission.
Area under the curve for severity and duration of hypotension (see the Materials and Methods).
Development of AKI
Moderate to severe (KDIGO stages 2–3) AKI occurred less frequently in the low-risk patients versus high-risk patients within 24 hours (14.3% versus 29.1%) and within 1 week (25.7% versus 51.7%) of ICU admission. Baseline characteristics of patients with and without AKI in the low-risk and high-risk groups are shown in Tables 2 and 3. Increased age and body mass index, presence of hypotension, sepsis, various comorbidities, and decreased reference eGFR were all associated with AKI in both low-risk and high-risk groups. Surgical patients were at decreased risk in patients with respiratory or cardiovascular organ failures, whereas no such relationship was observed in the low-risk group. In Table 4 we present the results of two multivariable regression models of risk factors for AKI in low-risk and high-risk patients. Overall, risk factors and the size of effects are very similar between groups. Furthermore, we found several statistically significant interactions between risk group and the considered risk factors (Supplemental Table 1).
Table 2.
Baseline characteristics in low-risk patients for AKI (−) and AKI (+)
| Characteristica | AKI− (n=13,379) | AKI+ (n=4637) | All (n=18,016) | P Valueb |
|---|---|---|---|---|
| Age, yr | <0.001 | |||
| 18–44 | 3138 (23.5) | 529 (11.4) | 3667 (20.4) | |
| 45–64 | 5057 (37.8) | 1581 (34.1) | 6638 (36.9) | |
| 65–74 | 2281 (17.1) | 1008 (21.7) | 3289 (18.3) | |
| ≥75 | 2898 (21.7) | 1519 (32.8) | 4417 (24.5) | |
| Men | 7518 (56.2) | 2516 (54.3) | 10,034 (55.7) | 0.02 |
| Race | 0.01 | |||
| White | 10,570 (79) | 3596 (77.6) | 14,166 (78.6) | |
| Black | 1099 (8.2) | 367 (7.9) | 1466 (8.1) | |
| Other | 1710 (12.8) | 674 (14.5) | 2384 (13.2) | |
| BMI, kg/m2 (n=12,222) | 26.3 (22.9–30.1) | 28.1 (24.2–33.2) | 26.8 (23.3–31.1) | <0.001 |
| Comorbid condition | ||||
| Diabetes | 830 (6.2) | 387 (8.3) | 1217 (6.8) | <0.001 |
| Cardiac disease | 1049 (7.8) | 494 (10.7) | 1543 (8.6) | <0.001 |
| Chronic renal disease | 217 (1.6) | 100 (2.2) | 317 (1.8) | 0.02 |
| History of hypertension | 4158 (31.1) | 1766 (38.1) | 5924 (32.9) | <0.001 |
| Multiple comorbidity | 4018 (30) | 1651 (35.6) | 5669 (31.5) | <0.001 |
| Surgical admission (n=16,730) | 6152 (49.6) | 2134 (49.2) | 8286 (49.5) | 0.62 |
| Creatinine, mg/dl | ||||
| Known baseline (n=6,835) | 0.9 (0.7–1.1) | 1 (0.7–1.3) | 0.9 (0.7–1.1) | <0.001 |
| Hospital admission (n=17,201) | 0.9 (0.7–1.1) | 1.1 (0.8–1.7) | 0.9 (0.7–1.2) | <0.001 |
| Reference | 0.8 (0.7–1) | 0.9 (0.8–1.1) | 0.8 (0.7–1) | <0.001 |
| Reference eGFR, ml/min per 1.73 m2 | ||||
| <30 | 200 (1.5) | 144 (3.1) | 344 (1.9) | <0.001 |
| 30–60 | 982 (7.3) | 502 (10.8) | 1484 (8.2) | |
| >60 | 12,191 (91.2) | 3991 (86.1) | 16,182 (89.9) | |
| APS-III Score (n=17,878)c | 36 (26–48) | 51 (40–65) | 40 (29–53) | <0.001 |
| Severity of hypotensionc,d | 0 (0–0) | 0 (0–0) | 0 (0–0) | <0.001 |
| Vasopressor usec | N/A | N/A | N/A | N/A |
| Mechanical ventilationc | N/A | N/A | N/A | N/A |
| Suspected sepsisc | 622 (4.6) | 475 (10.2) | 1097 (6.1) | <0.001 |
| Nephrotoxic drugs | ||||
| ACEI/ARB | 1613 (12.1) | 503 (10.8) | 2116 (11.7) | 0.03 |
| Vancomycin | 787 (5.9) | 413 (8.9) | 1200 (6.7) | <0.001 |
| Aminoglycoside | 164 (1.2) | 100 (2.2) | 264 (1.5) | <0.001 |
| Other antibiotics | 612 (4.6) | 224 (4.8) | 836 (4.6) | 0.47 |
| Calcineurin inhibitor | 314 (2.3) | 123 (2.7) | 437 (2.4) | 0.24 |
| Nonsteroidal | 3286 (24.6) | 1166 (25.1) | 4452 (24.7) | 0.43 |
| Diuretics | 1828 (13.7) | 1077 (23.2) | 2905 (16.1) | <0.001 |
| Other nephrotoxic drugs | 408 (3) | 123 (2.7) | 531 (2.9) | 0.17 |
Data are presented as n (%) or median (Q1–Q3) unless otherwise indicated. BMI, body mass index; APS-III, acute physiology score; N/A, not applicable; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICU, intensive care unit.
Missing values for age (five patients) and sex (one patient); otherwise the n for each variable is provided in parentheses.
P value for the comparison of AKI− and AKI+.
Measured within 24 hours after ICU admission.
Area under the curve for severity and duration of hypotension (see the Materials and Methods).
Table 3.
Baseline characteristics in high-risk patients for AKI (−) and AKI (+)
| Characteristica | AKI− (n=10,684) | AKI+ (n=11,452) | All (n=22,136) | P Valueb |
|---|---|---|---|---|
| Age, yr | <0.001 | |||
| 18–44 | 2742 (25.7) | 1576 (13.8) | 4318 (19.5) | |
| 45–64 | 3938 (36.9) | 4489 (39.2) | 8427 (38.1) | |
| 65–74 | 1946 (18.2) | 2604 (22.7) | 4550 (20.6) | |
| ≥75 | 2048 (19.2) | 2782 (24.3) | 4830 (21.8) | |
| Men | 6265 (58.6) | 6618 (57.8) | 12,883 (58.2) | 0.2 |
| Race | 0.001 | |||
| White | 8527 (79.8) | 8930 (78) | 17,457 (78.9) | |
| Black | 739 (6.9) | 813 (7.1) | 1552 (7) | |
| Other | 1418 (13.3) | 1709 (14.9) | 3127 (14.1) | |
| BMI, kg/m2 (n=21,149) | 25.3 (22.3–28.9) | 28 (24.3–33) | 26.6 (23.3–31) | <0.001 |
| Comorbid condition | ||||
| Diabetes | 480 (4.5) | 792 (6.9) | 1272 (5.7) | <0.001 |
| Cardiac disease | 757 (7.1) | 1229 (10.7) | 1986 (9) | <0.001 |
| Chronic renal disease | 125 (1.2) | 171 (1.5) | 296 (1.3) | 0.04 |
| History of hypertension | 2756 (25.8) | 3979 (34.7) | 6735 (30.4) | <0.001 |
| Multiple comorbidity | 2879 (26.9) | 4325 (37.8) | 7204 (32.5) | <0.001 |
| Surgical admission (n=20,766) | 6661 (66.8) | 6792 (62.9) | 13,453 (64.8) | <0.001 |
| Creatinine, mg/dl | ||||
| Known baseline (n=8787) | 0.9 (0.7–1.1) | 0.9 (0.7–1.2) | 0.9 (0.7–1.1) | <0.001 |
| Hospital admission (n=20,071) | 0.9 (0.7–1.1) | 1.1 (0.8–1.6) | 1 (0.8–1.3) | <0.001 |
| Reference | 0.8 (0.7–1) | 0.9 (0.8–1.1) | 0.9 (0.7–1) | <0.001 |
| Reference eGFR, ml/min per 1.73 m2 | ||||
| <30 | 131 (1.2) | 284 (2.5) | 415 (1.9) | <0.001 |
| 30–60 | 727 (6.8) | 1225 (10.7) | 1952 (8.8) | |
| >60 | 9815 (92.0) | 9941 (86.8) | 19,756 (89.3) | |
| APS-III score (n=22,119)c | 57 (42–76) | 78 (58–99) | 67 (48–89) | <0.001 |
| Severity of hypotensionc,d | 0 (0–3.2) | 0 (0–10.5) | 0 (0–6.5) | <0.001 |
| Vasopressor usec | 3336 (31.2) | 5224 (45.6) | 8560 (38.7) | <0.001 |
| Mechanical ventilationc | 9897 (92.6) | 10,504 (91.7) | 20,401 (92.2) | 0.01 |
| Suspected sepsisc | 924 (8.6) | 2153 (18.8) | 3077 (13.9) | <0.001 |
| Nephrotoxic drugs | ||||
| ACEI/ARB | 414 (3.9) | 536 (4.7) | 950 (4.3) | 0.003 |
| Vancomycin | 1197 (11.2) | 2023 (17.7) | 3220 (14.5) | <0.001 |
| Aminoglycoside | 164 (1.5) | 312 (2.7) | 476 (2.2) | <0.001 |
| Other antibiotics | 362 (3.4) | 618 (5.4) | 980 (4.4) | <0.001 |
| Calcineurin inhibitor | 579 (5.4) | 1158 (10.1) | 1737 (7.8) | <0.001 |
| Nonsteroidal | 1569 (14.7) | 1976 (17.3) | 3545 (16) | <0.001 |
| Diuretics | 1399 (13.1) | 2343 (20.5) | 3742 (16.9) | <0.001 |
| Other nephrotoxic drugs | 723 (6.8) | 1159 (10.1) | 1882 (8.5) | <0.001 |
Data are presented as n (%) or median (Q1–Q3) unless otherwise indicated. BMI, body mass index; APS-III, acute physiology score; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICU, intensive care unit.
Missing values for age (11 patients) and sex (1 patient); otherwise the n for each variable is provided in parentheses.
P value for the comparison of AKI− and AKI+.
Measured within 24 hours after ICU admission.
Area under the curve for severity and duration of hypotension (see the Materials and Methods).
Table 4.
Multivariable regression models of risk factors for AKI in low-risk and high-risk patients
| Outcome: AKI+ | Low Risk (n=16,724) | High Risk (n=20,755) | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (5 yr) | 1.11 (1.1 to 1.13) | <0.001 | 1.10 (1.09 to 1.11) | <0.001 |
| Men | 1.04 (0.97 to 1.12) | 0.26 | 1.10 (1.03 to 1.16) | 0.002 |
| Race | 0.001 | <0.001 | ||
| Black versus white | 1.09 (0.95 to 1.25) | 0.22 | 1.18 (1.05 to 1.32) | 0.01 |
| Other versus white | 1.22 (1.09 to 1.36) | <0.001 | 1.30 (1.19 to 1.43) | <0.001 |
| Diabetes | 1.23 (1.06 to 1.43) | 0.01 | 1.08 (0.95 to 1.24) | 0.26 |
| Cardiac disease | 1.02 (0.88 to 1.17) | 0.83 | 0.95 (0.84 to 1.06) | 0.35 |
| Chronic renal disease | 0.91 (0.7 to 1.19) | 0.5 | 0.80 (0.62 to 1.03) | 0.08 |
| History of hypertension | 1.05 (0.96 to 1.15) | 0.29 | 1.15 (1.07 to 1.23) | <0.001 |
| Multiple comorbidities | 1.02 (0.92 to 1.12) | 0.75 | 1.28 (1.19 to 1.39) | <0.001 |
| Surgical admission | 1.11 (1.03 to 1.2) | 0.004 | 0.96 (0.9 to 1.02) | 0.2 |
| Reference eGFR, ml/min per 1.73 m2 | <0.001 | 0.004 | ||
| <30 versus >60 | 1.65 (1.31 to 2.09) | <0.001 | 1.37 (1.1 to 1.72) | 0.01 |
| [30–60] versus >60 | 1.15 (1.02 to 1.31) | 0.03 | 1.12 (1.01 to 1.25) | 0.04 |
| Severity of hypotensiona | 1.007 (1.005– to 1.009) | <0.001 | 1.007 (1.006– to 1.008) | <0.001 |
| Suspected sepsis | 2.1 (1.83 to 2.42) | <0.001 | 2.21 (2.02 to 2.43) | <0.001 |
| ACEI/ARB | 0.75 (0.67 to 0.85) | <0.001 | 0.98 (0.85 to 1.13) | 0.76 |
| Vancomycin | 1.3 (1.13 to 1.49) | <0.001 | 1.23 (1.13 to 1.34) | <0.001 |
| Aminoglycoside | 1.66 (1.27 to 2.18) | <0.001 | 1.39 (1.12 to 1.71) | 0.002 |
| Other antibiotics | 0.96 (0.81 to 1.14) | 0.66 | 1.21 (1.05 to 1.4) | 0.01 |
| Calcineurin inhibitor | 1.12 (0.88 to 1.41) | 0.36 | 1.84 (1.61 to 2.09) | <0.001 |
| Nonsteroidal drugs | 0.97 (0.89 to 1.06) | 0.51 | 0.96 (0.88 to 1.04) | 0.31 |
| Diuretics | 1.56 (1.42 to 1.71) | <0.001 | 1.42 (1.31 to 1.54) | <0.001 |
| Other nephrotoxic drugs | 0.9 (0.73 to 1.12) | 0.36 | 1.33 (1.18 to 1.5) | <0.001 |
OR, odds ratio; 95% CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Area under the curve for severity and duration of hypotension (see the Materials and Methods).
Survival
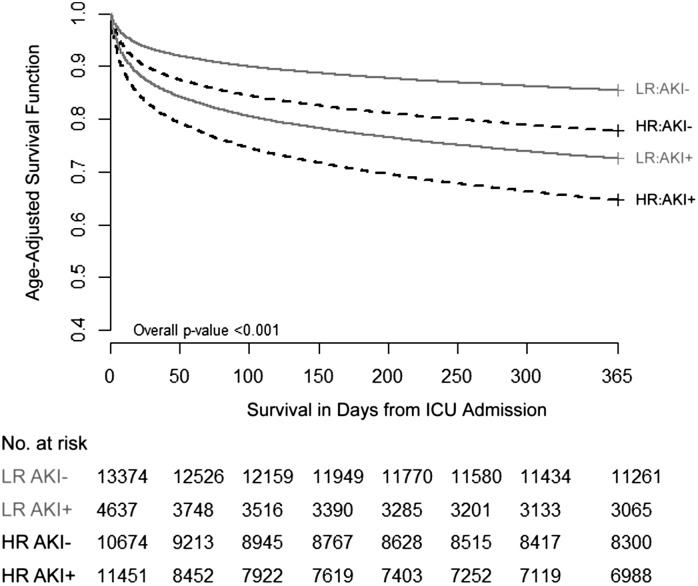
In both low- and high-risk patients, after adjusting for age, those developing AKI had increased risk of death by hospital discharge and by 30, 90, and 365 days compared with those not developing AKI (Figure 2, Table 5). Overall, as expected, survival was better in low-risk compared with high-risk patients (Table 5); death by hospital discharge and 1 year was 6.6% and 20.5% for low-risk patients compared with 18.1% and 30.9% for high-risk patients (P<0.001). However, for low-risk patients developing AKI any time in the first week after ICU admission, mortality at hospital discharge was 15.3% compared with 3.6% for those without AKI. For high-risk patients, mortality at hospital discharge was 23.9% for those developing AKI versus 11.9% for those who did not. Thus, mortality rates increase 4-fold with AKI in low-risk patients (crude relative risk, 4.3; 95% confidence interval, 3.8 to 4.8) but only double in high-risk patients (crude relative risk, 2; 95% confidence interval, 1.9 to 2.1). Differences in relative risks for mortality persist at 30 days (3.2 versus 1.9) but attenuate by 1 year (2.1 versus 1.8). Additional survival data (stratified by KDIGO stage) are provided in Supplemental Figure 1. Although the 1-year age-adjusted survival for low-risk patients with AKI was better than for high-risk patients with AKI (Figure 2), the adjusted odds of hospital mortality associated with AKI was actually greater for low-risk patients (odds ratio, 2.99; 95% confidence interval, 2.62 to 3.41) than for those with respiratory or cardiovascular failures (odds ratio, 1.19; 95% confidence interval, 1.09 to 1.3). After adjusting for multiple risk factors, there was strong evidence of a difference in effect of AKI on hospital mortality (based on association) between low-risk and high-risk patients (P<0.001 for the interaction) (Table 6).
Figure 2.
Age-adjusted 1-year survival for four groups: LR AKI−, LR AKI+, HR AKI−, and HR AKI+. Age was available in 40,136 patients. HR, high risk; LR, low risk.
Table 5.
Patient outcomes by AKI status
| Characteristic | AKI− | AKI+ | AKI+ versus AKI- | P Valuea |
|---|---|---|---|---|
| Low-risk group | ||||
| Patients, n | 13,379 | 4637 | ||
| Mortality, % | ||||
| Hospital | 3.6 | 15.3 | 4.3 (3.8 to 4.8) | <0.001 |
| 30 d | 4.9 | 15.9 | 3.2 (2.9 to 3.6) | <0.001 |
| 90 d | 8.6 | 23.5 | 2.7 (2.5 to 3.0) | <0.001 |
| 365 d | 15.8 | 33.9 | 2.1 (2.0 to 2.3) | <0.001 |
| Length of stay, d | ||||
| ICU | 2 (2–3) | 4 (3–7) | N/A | <0.001 |
| Hospital | 7 (4–12) | 12 (7–21) | N/A | <0.001 |
| High-risk group | ||||
| Patients, n | 10,684 | 11,452 | ||
| Mortality, % | ||||
| Hospital | 11.9 | 23.9 | 2.0 (1.9 to 2.1) | <0.001 |
| 30-day | 12.2 | 22.7 | 1.9 (1.8 to 2.0) | <0.001 |
| 90-day | 15.8 | 30.2 | 1.9 (1.8 to 2.0) | <0.001 |
| 365-day | 22.2 | 39.0 | 1.8 (1.7 to 1.8) | <0.001 |
| Length of stay, d | ||||
| ICU | 3 (2–6) | 6 (4–13) | N/A | <0.001 |
| Hospital | 9 (6–17) | 15 (9–26) | N/A | <0.001 |
Data are presented as median (Q1-Q3) or relative risks (95% CIs) unless otherwise indicated. d, days; N/A, not applicable.
P value is for the comparison of AKI− and AKI+.
Table 6.
Multivariable regression model for hospital mortality (n=37,342)
| Outcome: Hospital Mortality | OR (95% CI) | P Valuea |
|---|---|---|
| Risk group* AKI | ||
| LR AKI− versus HR AKI− | 0.5 (0.44 to 0.56) | <0.001 |
| HR AKI+ versus HR AKI− | 1.19 (1.09 to 1.3) | <0.001 |
| LR AKI+ versus HR AKI− | 2.52 (2.15 to 2.94) | <0.001 |
| Age (by 5 yr) | 1.1 (1.09 to 1.11) | <0.001 |
| Race | ||
| Black versus white | 0.95 (0.82 to 1.09) | 0.43 |
| Other versus white | 1.97 (1.81 to 2.15) | <0.001 |
| Cardiac disease | 1.22 (1.09 to 1.36) | <0.001 |
| Surgical admission | 0.54 (0.5 to 0.58) | <0.001 |
| APS-III derived score | 1.031 (1.029 to 1.032) | <0.001 |
| Severity of hypotensionb | 1.008 (1.007 to 1.009) | <0.001 |
| Suspected sepsis | 1.1 (1 to 1.21) | 0.04 |
OR (95% CI) for LR AKI+ versus LR AKI−: 2.99 (2.62 to 3.41). LR, low risk; HR, high risk; OR, odds ratio; 95% CI, 95% confidence interval; APS-III, acute physiology score.
P value for interaction between risk group and AKI <0.001.
Area under the curve for severity and duration of hypotension (see the Materials and Methods).
LOS
Additional outcomes are reported in Table 5. Although the overall median hospital LOS was 4 days shorter in the low-risk group, the development of AKI was associated with roughly a 2-fold increase in ICU and hospital LOS in both low- and high-risk patients.
Timing of AKI in Relation to Other Organ Failures
Although AKI, when it occurred, occurred after (or on the same day) as provision of mechanical ventilation or vasopressors in all high-risk patients, only 5.7% of low-risk patients received these therapies before AKI (after ICU day 1). Characteristics of these patients are provided in Supplemental Table 2. Indeed, 64.4% of low-risk patients developing AKI never received mechanical ventilation or vasopressors.
Discussion
Very little is known about the susceptibilities and consequences of AKI in ICU patients without other organ failures present at the time of ICU admission. Prior studies have shown that most AKI in critically ill patients occurs in the setting of multiorgan failure (15) and thus, most efforts to understand and prevent AKI in critically ill individuals have focused on high-risk ICU patients, typically those with multiorgan failure. Although this approach will identify the majority of patients developing AKI, it has two important shortcomings. First, patients with isolated AKI or those with AKI as the first organ failure may be more amenable to therapy. Although multiorgan failure has been the focus of intense research for >30 years, no approved therapies exist. By contrast, isolated AKI might be more likely to be due to more specific and treatable conditions. Second, AKI is sometimes the first manifestation of multiorgan failure and focusing only on patients with established organ failures involving systems other than the kidney will automatically result in late detection of AKI in these patients. Indeed, we note that traditional study designs for sepsis specify enrollment of patients already manifesting organ failures and indeed would exclude many patients with impending AKI (1).
Similar limitations can be seen in studies evaluating novel biomarkers for AKI that have specifically focused on high-risk patients (16). Ironically, although many more events will be seen in high-risk patients, our data suggest that the relative effect of AKI on survival is actually greatest in low-risk patients. Indeed, the absolute increase in risk of death is almost identical in both groups. Patients without other organ failures, by virtue of their lower clinical complexity, may be easier to prevent from developing AKI in the first place. This leads to the supposition that efforts to curtail AKI in critically ill patients might be more effective if applied to low-risk patients, or at least that low-risk patients should not be excluded. It is further notable that both ICU and hospital LOS is approximately double in AKI-positive compared with AKI-negative patients regardless of risk group.
To our surprise, patients developing AKI in low- and high-risk groups had very similar exposures and susceptibilities (Tables 2 and 3). Obviously respiratory failure and circulatory shock were present only in high-risk patients (by definition), but other risk factors appeared very similar across both groups. To our knowledge, this is the first large-scale investigation of AKI events and outcomes in low-risk ICU patients. We have previously shown that patients with low-risk community-acquired pneumonia, including those not admitted to ICU, are still at high risk for AKI and the association with decreased survival is quite dramatic (1). In addition, Joannidis and Metnitz reported that AKI contributed more to mortality in patients with lower baseline severity of illness (17). Indeed, these prior findings agree well with our current results.
Our study also demonstrates that the severity of AKI is a better determinant of long-term outcomes than the initial organ (cardiovascular and respiratory) failure for high-risk patients. Both the low- and high-risk groups that develop AKI have mortality rates between 34% and 39% at 1 year. These findings suggest that significant efforts should be made to prevent any patient, irrespective of risk, from developing AKI or for patients with mild AKI (stage 1), from progressing in AKI severity. The KDIGO guidelines provide recommendations for management of high-risk patients and those with mild AKI such as removing nephrotoxic drugs, avoiding contrast-related procedures, and monitoring serum creatinine and urine output (2). Our results suggest that patients with presumed sepsis, advanced age, and underlying renal dysfunction are at increased risk for AKI whether or not they have other organ failures at the time of ICU admission. Although proper management of AKI will improve outcomes for both low- and high-risk patients, there may be additional benefit to low-risk patients because their short-term outcomes (hospital mortality and mortality at 30 days from ICU) are more significantly impacted by AKI. This also emphasizes the need for care after ICU discharge, in an attempt to improve patient outcomes and assess for potential recovery of renal function. Unfortunately, clinical follow-up for AKI survivors is low (18).
Opportunities for improvement in patient care exist with identification and management of low- and high-risk AKI patients. Automated clinical surveillance systems are effective for a variety of clinical conditions including the identification of sepsis (19), adverse drug reactions (20,21), and more recently, AKI (22,23). These surveillance systems have assisted with faster identification of events compared with usual care that has been shown to improve short-term progression of AKI severity (22,24,25). Future studies are needed to determine the effect of clinical surveillance systems used to detect AKI on long-term recovery of renal function, as well as how these systems can be further refined to enhance the detection and management of AKI.
Our study has limitations. Although we examined a very large cohort, the patients are all from a single medical center (although it includes multiple ICUs in different hospitals). Because this is the first investigation of its kind, it is difficult to know how generalizable our results will be. However, the close agreement with our prior work in patients with pneumonia (1) enrolled from 28 different sites gives some confidence that our results are not unique to this medical center. Our overall event rates and outcomes agree well with recent epidemiologic studies of AKI from around the world (26–28). In addition, because many low-risk patients may not be admitted to the ICU, our study could apply to a wider group of patients than we reported on. Second, as a practical matter for analysis, we chose to dichotomize AKI rather than consider all three stages. Our choice to consider only moderate to severe AKI (KDIGO stages 2–3) as events and lump stage 1 AKI together with no AKI is arguable. We note that although many studies have demonstrated the importance of even mild fluctuations in renal function (1,8,26), the most consistent relationships with adverse outcomes are seen with more severe disease (8,29). Indeed, some data suggest that some patients without clinical evidence for AKI may still have subclinical injury as detected by biomarkers and supported by changes in long-term outcomes including death and dialysis (30). As a sensitivity analysis, we examined all stages of AKI in both low- and high-risk patients (Supplemental Figure 1) and our primary conclusions remain unchanged.
In conclusion, ICU patients without respiratory failure or circulatory shock may well be at low risk for AKI. However, when these patients develop AKI, a nearly identical effect on hospital and ICU LOS is observed and the relative changes in mortality associated with AKI are actually greater in this population. Quality improvement and future studies of AKI should not exclude low-risk ICU patients without careful consideration.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01-DK070910 and R01-DK083961 to J.A.K.) and the National Center for Research Resources (NCRR) (KL2 RR024154 to R.M.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIDDK, or NCRR.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03200314/-/DCSupplemental.
References
- 1.Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, Angus DC, Kellum JA, Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators : Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 77: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease Improving Global Outcomes Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 3.MacLeod A: NCEPOD report on acute kidney injury-must do better. Lancet 374: 1405–1406, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG, Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Yount R, Vries J, Councill C: The Medical Archival System: An information retrieval system based on distributed parallel. Inf Process Manage 27: 379–389, 1991 [Google Scholar]
- 6.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A: The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100: 1619–1636, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup : Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580–637, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA: RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care 10: R73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, Bellomo R, Kellum JA, AKI6 investigators : A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant 25: 3911–3918, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Pai AB, Mason DL: Acute kidney injury. In: Drug-Induced Diseases: Prevention, Detection and Management, 2nd Ed., edited by Tisdale JE, Miller DA, Bethesda, MD, American Society of Health-System Pharmacists, 2010, pp 853–871 [Google Scholar]
- 11.Perazella MA, Markowitz GS: Drug-induced acute interstitial nephritis. Nat Rev Nephrol 6: 461–470, 2010 [DOI] [PubMed] [Google Scholar]
- 12.UpToDate : Drug Information, edited by Post TW, Waltham, MA, UpToDate, 2013 [Google Scholar]
- 13.Micromedex Healthcare Series : DRUGDEX System, Greenwood Village, CO, Truven Health Analytics, 2013 [Google Scholar]
- 14.Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET: Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract 2012: 691013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F: Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joannidis M, Metnitz PGH: Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin 21: 239–249, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein SL, Jaber BL, Faubel S, Chawla LS, Acute Kidney Injury Advisory Group of American Society of Nephrology : AKI transition of care: A potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol 8: 476–483, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Sawyer AM, Deal EN, Labelle AJ, Witt C, Thiel SW, Heard K, Reichley RM, Micek ST, Kollef MH: Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med 39: 469–473, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Kane-Gill SL, Visweswaran S, Saul MI, Wong AKI, Penrod LE, Handler SM: Computerized detection of adverse drug reactions in the medical intensive care unit. Int J Med Inform 80: 570–578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Classen DC, Resar R, Griffin F, Federico F, Frankel T, Kimmel N, Whittington JC, Frankel A, Seger A, James BC: ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood) 30: 581–589, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Colpaert K, Hoste EA, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, Decruyenaere J: Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 40: 1164–1170, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Matheny ME, Miller RA, Ikizler TA, Waitman LR, Denny JC, Schildcrout JS, Dittus RS, Peterson JF: Development of inpatient risk stratification models of acute kidney injury for use in electronic health records. Med Decis Making 30: 639–650, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuperman GJ, Teich JM, Tanasijevic MJ, Ma’Luf N, Rittenberg E, Jha A, Fiskio J, Winkelman J, Bates DW: Improving response to critical laboratory results with automation: Results of a randomized controlled trial. J Am Med Inform Assoc 6: 512–522, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etchells E, Adhikari NKJ, Cheung C, Fowler R, Kiss A, Quan S, Sibbald W, Wong B: Real-time clinical alerting: Effect of an automated paging system on response time to critical laboratory values—a randomised controlled trial. Qual Saf Health Care 19: 99–102, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 27.US Renal Data System : Acute kidney injury. In: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009, pp 119–130 [Google Scholar]
- 28.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS: Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 9: 12–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling C-R, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57: 1752–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.