Figure 10.
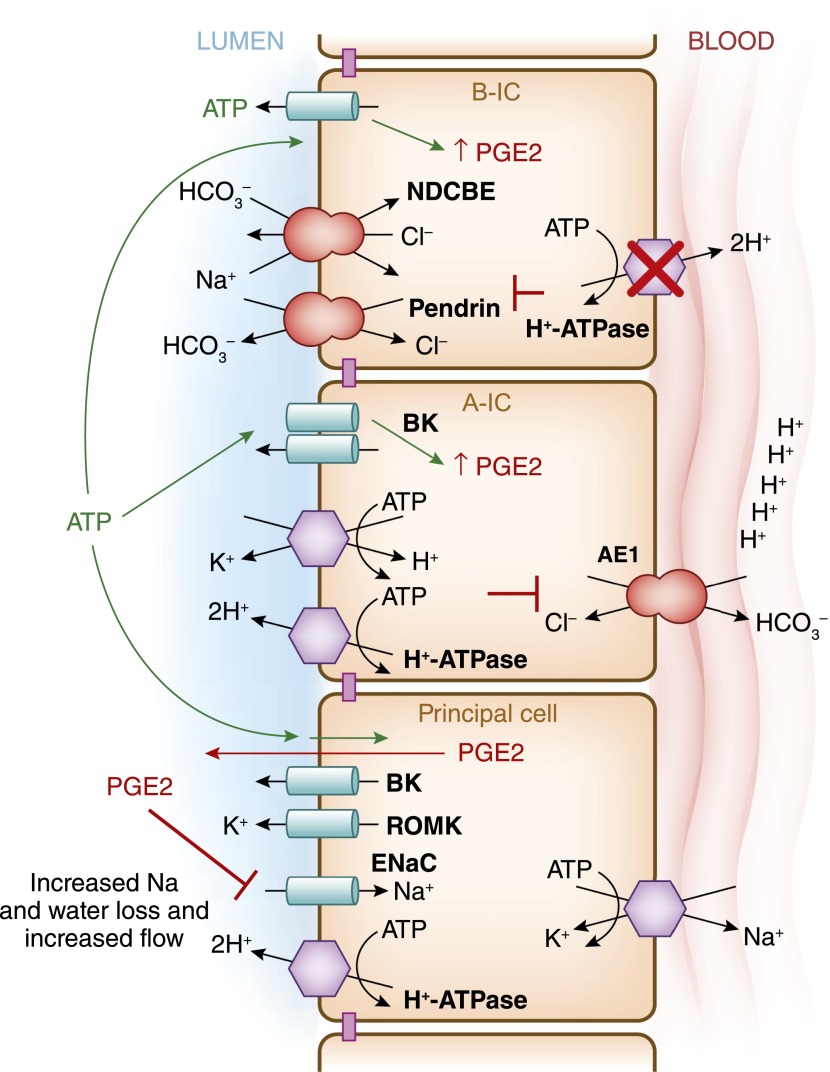
Intercalated cells are necessary to maintain body fluid and electrolyte balance. This model summarizes the recent findings by Gueutin and colleagues (35), which showed how H+-ATPase dysfunction in type B-IC leads to the urinary water and sodium losses in patients with distal renal tubule acidosis. This group studied mice with significantly decreased H+-ATPase activity in the collecting duct intercalated cells due to knockout of the B1 of the H+-ATPase subunit (ATP6V1B1–/–mice). The specific knockout of the B1 subunit does not disturb H+-ATPase expression in the proximal tubule. These animals presented with a significant urinary loss of NaCl, revealing impaired function of epithelial sodium channel (ENaC) in principal cells, as well as decreased pendrin and NDCBE function in type B-IC in the cortical collecting duct. These animals had an upregulation of ENaC in the medulla. High levels of prostaglandin E2 (PGE2) and ATP were detected in the urine of these animals. When PGE2 was normalized using pharmacologic agents these animals also normalized their ENaC levels in the cortex, and they had improved polyuria and hypokalemia. When the H+-ATPase was inactivated in type B-IC, it resulted in ATP release with the subsequent increases in PGE2 release from these cell as well.