Fig. 4.
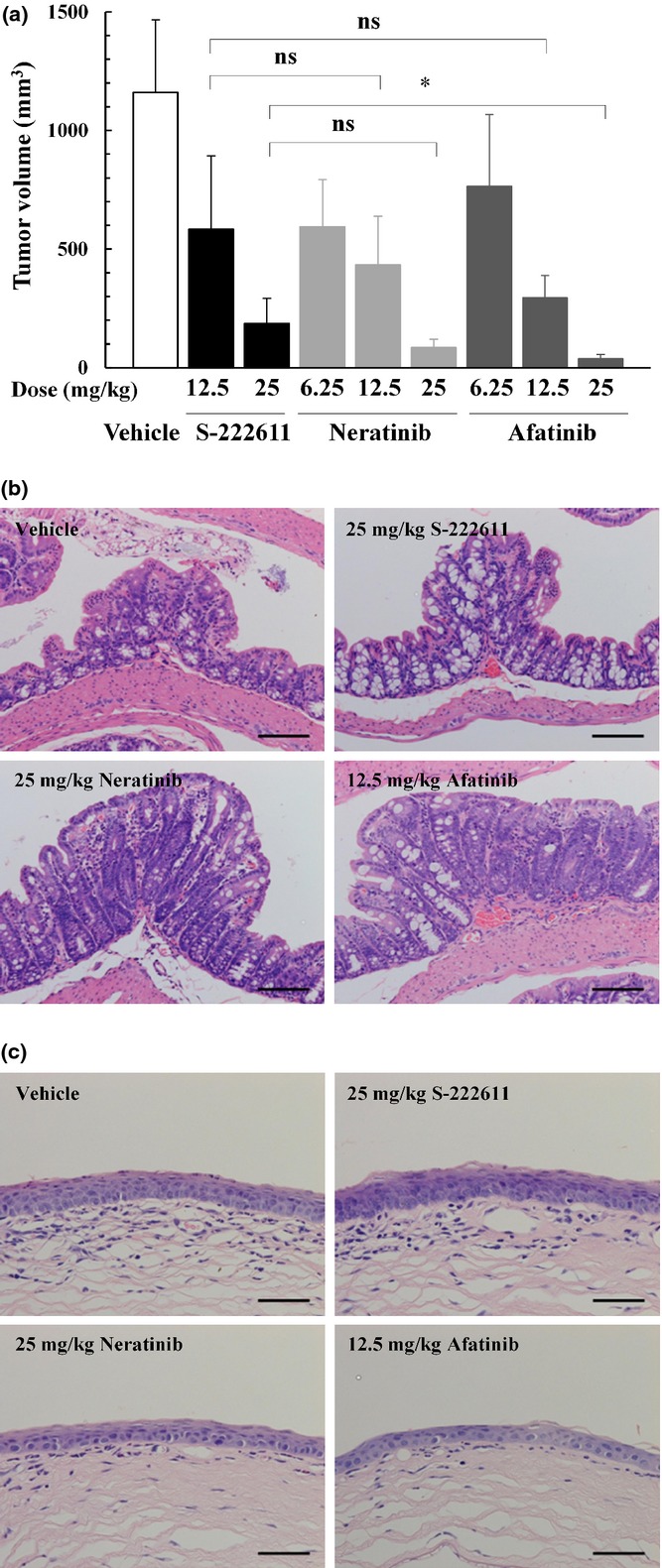
In vivo antitumor activity and toxicity profile of S-222611, neratinib and afatinib in the NCI-N87 model. (a) Human gastric cancer cells, NCI-N87, were implanted subcutaneously into nude mice. After randomization at 3 days after implantation (n = 9), vehicle or multiple doses of S-222611, neratinib or afatinib were orally administered daily for 21 days. Mean tumor volume with SD at the last measurement (the day following the last administration) is represented in each bar graph. ns: P ≥ 0.05, *: P < 0.05 (Welch's t-test with Dunn–Šidák multiple test correction). (b,c) Representative histopatholgical images of the colon (b) and eyeball (c) of mice treated with vehicle, S-222611 (25 mg/kg), neratinib (25 mg/kg) and afatinib (12.5 mg/kg). Regeneration in the colonic epithelium (b) and atrophy in the corneal epithelium were observed in neratinib-treated and afatinib-treated mice. No remarkable changes were observed in vehicle-treated and S-222611-treated mice. Stained with H&E. Bar: 100 μm.
