Abstract
Background:
Drug eruptions range from transient erythema to the life threatening severe cutaneous adverse reactions (SCAR) that encompass Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms complex (DRESS).
Aims and Objectives:
To study the clinical and epidemiological aspects of cutaneous adverse drug reactions (CADR).
Materials and Methods:
Ethical clearance was obtained from the institutional ethics committee. All patients admitted in the Dermatology ward of our tertiary care hospital with CADR (those who fit in the category of probable or possible drug reaction as per WHO casuality assessment) from first September 2011 to 31st August 2012 were included in this cross sectional study after obtaining written informed consent. The drug reaction patterns observed in the study population were determined and the common offending drugs were identified.
Results:
In the study, population of males outnumbered females and the majority were between 46 and 60 years of age. The commonest reaction pattern observed was SJS- TEN spectrum of illness and aromatic anticonvulsants were the common offending drugs. Prompt withdrawal of the culprit drug and administration of systemic steroids with or without I/V Ig reverted the adverse reaction in all except one.
Conclusion:
Severe drug reactions predominated as the study population was comprised of inpatients of a tertiary referral centre. Though; previous authors had reported a mortality rate of up to 20% in DRESS, all our patients with this reaction pattern, responded well to treatment. The mortality rate among TEN cases was much lower than the previous reports. Early diagnosis, prompt withdrawal of the suspected drug, careful monitoring for development of complications and immediate intervention can improve the prognosis of CADR.
Keywords: Drug reaction, drug reaction with eosinophilia and systemic symptoms complex, epidemiology
What was known?
CADR range from mild cutaneous manifestations to life threatening SCAR. Early diagnosis of CADR is necessary for the effective management.
Introduction
Drug reactions are unwanted reactions of the body that occur following the administration of drugs and that are not characteristic of the desired pharmacodynamic effects.[1] They vary from transient erythema to severe forms (collectively designated as SCAR).[2] The pattern of drug reactions and the offending drugs show changing trends with the introduction of newer drugs. Drug reactions are most often under-reported and many questions regarding the pathogenesis are yet to be addressed. They can mimic viral exanthemes or neoplastic diseases or collagen vascular diseases. A high degree of suspicion is required to make a prompt diagnosis which is crucial in the management of CADR. In this study; we have tried to determine the different patterns of drug reactions and the offending drugs in patients who were admitted in the Dermatology ward of our institution with CADR during the one year study period.
Materials and Methods
Ethical clearance was obtained from the institutional ethics committee.
Inclusion criteria
Patients admitted in the Dermatology ward of our tertiary care hospital during the study period (from 1st September 2011 to 31st August 2012) with CADR, were classified as probable, possible or unlikely case of drug reaction, as per WHO definitions[3]. After obtaining written informed consent, only the probable and possible cases on causality assessment were included in the study (we can’t comment whether they were certain CADR, as drug rechallenge was not performed) DRESS, SJS, TEN and AGEP were considered as SCAR as per RegiSCAR group recommendations.[1].
Exclusion criteria
Patients who developed CADR following intake of homeo, ayurveda and indigenous medicines were excluded from the study.
A pre-set proforma (see appendix) was used to collect patients’ details including demographic data, detailed clinical history, past history and comorbidities. To determine the culprit drug, the drug history, temporal correlation with the drug, duration of skin lesions, time interval between the drug intake and onset of rash, morphology of drug eruption, associated mucosal or systemic involvement and improvement of lesions on withdrawl of drug were carefully analysed, In patients receiving multiple drugs, the most likely offending agent was identified on the basis of the type of reaction and the latent period between drug intake and the onset of reaction and this was further confirmed by the improvement on the withdrawal of the same. Blood and urine investigations, liver function test, renal function test, random blood sugar estimation and screening for retro infection were done in all. Chest radiography and ultrasound abdomen were done in relevant cases. Different types of drug reactions manifested in the study population were studied and the culprit drugs were noted.
Results
During the one year study period, 43 of 106 patients (40.6%) who attended our dermatology department with CADR required hospitalization. Fourteen patients (32.6% of total admissions due to CADR) were admitted through the emergency department. Of these 14, there were 10 cases of TEN, 2 of SJS and one each of AGEP and exfoliative dermatitis. Males outnumbered females (the male to female ratio being 1.5:1). There was a male preponderance in all types of drug reactions with the exception of DRESS and erythema multiforme (EM). Majority was in the 46-60 years age group with a decline in prevalence of drug reactions in those above 60 [Table 1]. The commonest reaction pattern [Table 2] observed was SJS-TEN (5 were SJS and 12 were TEN) [Figure 1] followed by maculopapular rash [Figure 2] and DRESS [Figure 3]. Commonest drug group producing reactions were aromatic anticonvulsants (20/43, 46.5%) followed by antibiotics and NSAIDs [Table 3]. Phenytoin was the commonest offender followed by carbamazepine. In 48% of SCAR cases, the culprit drug was aromatic anticonvulsants, whereas NSAIDs and antibiotics contributed to 32% of SCAR. Compared to phenytoin, carbamazepine was more commonly associated with SCAR (five out of eleven CADR produced by phenytoin and six out of eight reactions induced by carbamazepine were SCAR). Non-SCAR reactions in the study population were commonly induced by phenytoin (6), antibiotics (5) and NSAIDs (4). Fifty percent of non-SCAR reactions were caused by NSAIDs and antibiotics. One female patient presented with multiple lesions of FDE [Figure 4].
Table 1.
Age distribution in drug reactions

Table 2.
Drug reaction patterns
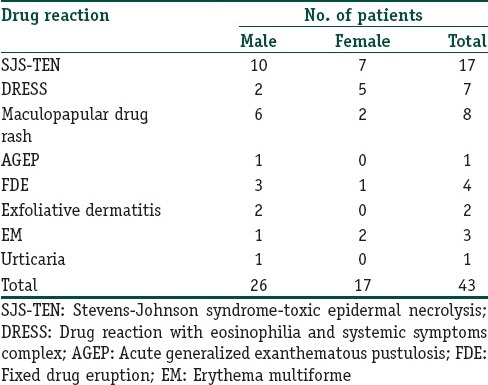
Figure 1.
Spots of toxic epidermal necrolysis
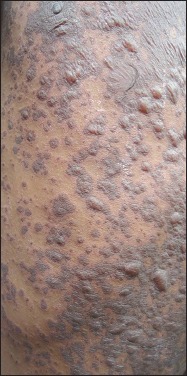
Figure 2.
Erythematous macules and papules of maculopapular drug rash
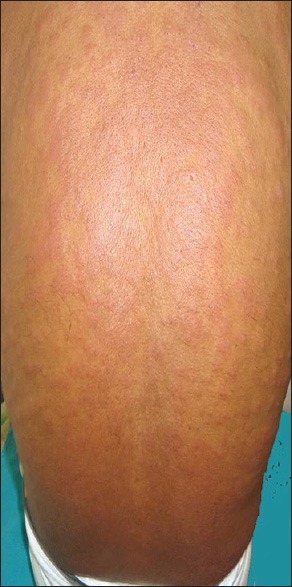
Figure 3.
Erythema, oedema and pustulation in drug reaction with eosinophilia and systemic symptoms complex
Table 3.
Drugs producing adverse reactions
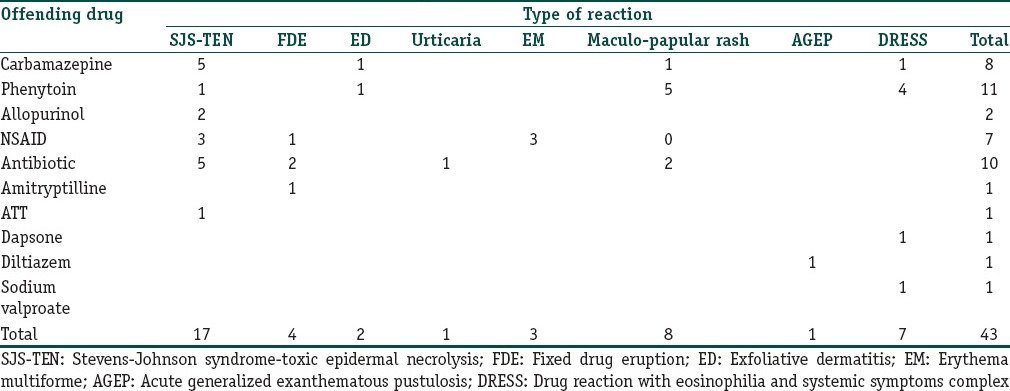
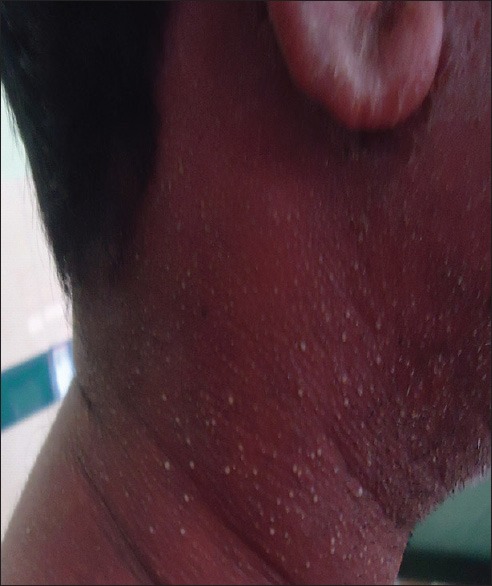
Figure 4.
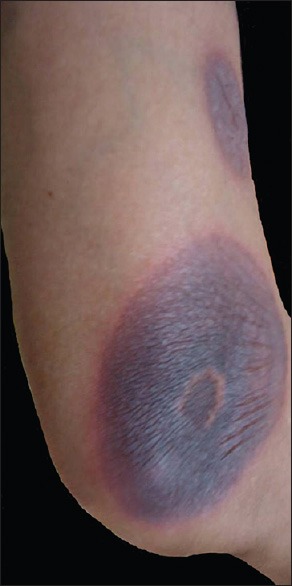
Well circumscribed, hyperpigmented lesions of fixed drug eruption
Latent period between the drug intake and the onset of symptoms varied from 12h to 21 days in drug reactions other than DRESS [Figure 5]. One patient who gave history of fixed eruption following etoricoxib, on re-exposure to the same drug developed similar lesion within 12 h. With antibiotics and NSAIDs, the usual time interval observed between the drug intake and the onset of adverse reaction was one to three days, whereas anticonvulsants produced reactions other than DRESS within a span of 12 to 21 days. In DRESS, this latent period varied from 21 to 90 days with an average of 37 days.
Figure 5.
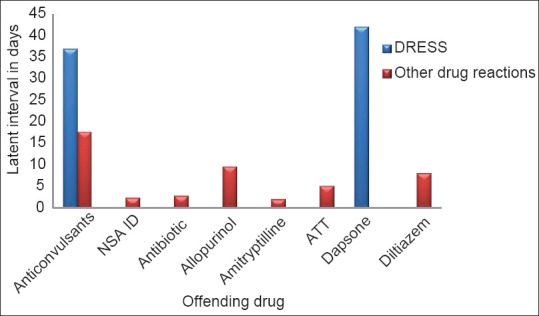
Time interval between drug intake and onset of reaction
Twelve of forty three (27.9%), developed reaction following drug intake for an infectious illness (antibiotics or NSAIDs).
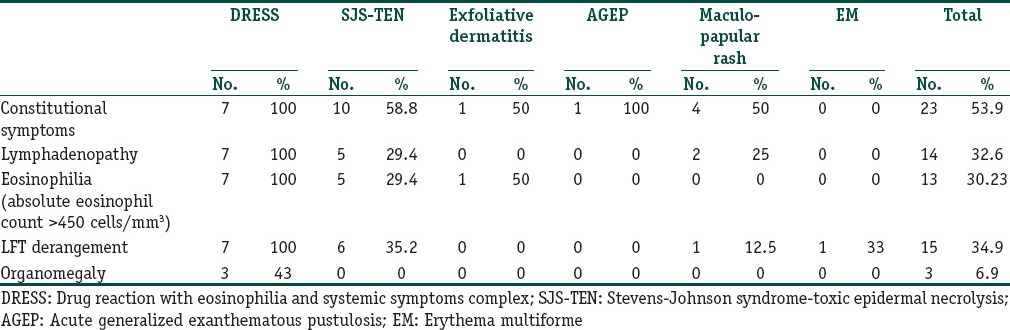
From Table 4, it is obvious that eosinophilia (eosinophil count above 450 cells/mm3) constitutional symptoms, lymphadenopathy and LFT derangement were more prevalent among severe drug reactions. All the DRESS patients in our study had systemic involvement in the form of LFT derangement. Two DRESS patients (after intake of carbamazepine and phenytoin respectively) had lung involvement in the form of pneumonitis. Though systemic involvement was more common in DRESS cases, they had relatively less severe skin and mucosal involvement (limited to scaling of lips in five patients and two patients had conjunctival congestion in addition). Facial erythema and oedema was more marked in phenytoin induced DRESS.
Table 4.
Extracutaneous manifestations in drug reactions
The SJS-TEN patient who had LFT derangement, manifested more severe mucocutaneous involvement. On the contrary, the patients with maculopapular drug rash and EM who developed LFT derangement had no other clinical differences, from their counterparts without liver function abnormality.
Comorbidities noted in our study population were hypertension (3 patients), diabtes mellitus (1 patient), interstitial nephritis (1 patient), cirrhosis liver (one patient) and coronary artery disease (1 patient). In addition one patient had multiple comorbidities (diabetes mellitus, hypertension and chronic obstructive pulmonary disease) and he succumbed to his illness. Except in the patient with coronary artery disease (who developed AGEP following intake of diltiazem prescribed for ventricular fibrillation) in all others drug reactions were precipitated by medications given for unrelated ailments. None of our patients had coexisting retro-infection or collagen vascular disease.
One patient who came to us with SJS following intake of etoricoxib gave history of reactions to multiple drugs (diclofenac, paracetamol and ketorolac) in the past. This was a 45-year old female, who was taking NSAIDs for arthritis of knee and ankle joints for the past 9 years. Three other patients in the study group gave history of drug reactions in the past and all these three developed reactions during the study period, on re-exposure to the same drug that had produced adverse reaction in the past (etoricoxib, doxycyclin and cefadroxyl respectively were the causative drugs).
None of our patients gave history of CADR in a close family member.
All our patients with drug rashes were treated with the withdrawal of the culprit drug and administration of 1-2 mg/kg body weight of prednisolone or dexamethasone equivalent. In addition, I/V Ig were given to SJS-TEN patients with severe skin and mucosal involvement. All except one patient, responded to this line of management. The lone patient who didn’t improve was a 67 year old male who suffered TEN following carbamazepine taken for post herpetic neuralgia. He had multiple comorbidities and he expired on the 6th post-admission day, thus producing a mortality rate of 8.3% (1/12) among the TEN cases of the study group.
Two of our DRESS patients, on tapering the steroids, developed rebound flare up but responded to a higher dose of steroids which was tapered more slowly. In all except these two, we were able to stop steroids within three weeks. Average duration of hospital stay was maximum for SJS-TEN patients and ranged from two to six weeks. For DRESS and AGEP this varied from one to two weeks. In all other drug reactions we were able to discharge the patients within a week. Though DRESS patients were at a higher risk for developing systemic involvement, once diagnosed we were able to manage them relatively easily (with withdrawal of the culprit drug and administration of systemic steroids).
The major complication observed in the study population was septicemia. Six of our patients developed this complication and all of them suffered from SJS-TEN spectrum of illness. One of the TEN patients, developed adult respiratory distress syndrome and required ventelatory care.
Discussion
A significant percentage of CADR patients who attended our department required inpatient care. This could be due to the fact that patients with more severe reactions seek medical aid in a tertiary referral centre. Analysis of data revealed that most of our patients were in the 46-60 years age group, in concordance with the observations made by many previous authors.[4,5] This is attributed to an increased use of medicines as age advances, leading to a heightened potential for drug to drug interactions and altered drug handling by the body.[6] The sharp decline after 60 could be due to the immunodeficiency associated with old age. There are studies that have documented a higher prevalence of drug reactions in the 20-40 age group.[6,7,8] The age disparity in various studies may be due to the regional variations in health care seeking behaviour of the population.[7] As in our study; male predominance was documented in many other studies.[7,8] Heizerling et al.,[9] and Pudukadan et al.,[6] reported a higher prevalence among females.
Time interval between the onset of drug intake and the manifestation of CADR and the pattern of reaction observed were used to determine the offending drug in patients on polypharmacy and we were able to identify the culprit drug in all our patients.
Time interval between the drug intake and the onset of reaction vary with the type of CADR. It ranges from less than a day in urticaria to three weeks to 3m in DRESS. Any drug can produce maculopapular rash at any time upto three weeks after the onset of drug intake. On re-exposure to the same drug, all these reactions can develop within 24 h.
All drugs are capable of producing any type of reaction in susceptible individuals but some drugs are more likely to induce certain reaction patterns and this can also give a clue regarding the likely causative drug. Aromatic anticonvulsants, antituberculous drugs, pencillins, cephalosporins, sulfa group of drugs, allopurinol and NSAIDs are more likely to produce SJS-TEN spectrum of illness. The drugs well known to produce DRESS are aromatic anticonvulsants, lamotrigine, minocycline, salazopyrine and dapsone. Urticaria is commonly produced by pencillins, angiotensin converting enzyme inhibitors (ACE inhibitors), aspirin and other NSAIDs. FDE is commonly associated with NSAIDs, tetracyclins, doxycyclin, cotrimoxazole, pencillins, cephalosporins and phenobarbitone, Exfoliative dermatitis is known to be induced by antibiotics, NSAIDs, dapsone, anticonvulsants etc., Macrolide antibiotics, hydroxychloroquin, and diltiazem are well known to induce AGEP.[10] The female patient who presented with multiple lesions of FDE gave a history of recurrent attacks of one or two FDE lesions for the past one year. A detailed drug history revealed that she was receiving amitryptilline and NSAID for peripheral neuropathy intermittently during the above mentioned period. With the appearance of FDE, she was asked to avoid NSAID which is commonly associated with FDE and was continued on amitryptilline. We advised her to stop amitryptilline and the present episode was managed with systemic steroids. She was relieved of the lesions and has remained symptom free to this date. Repeated exposures to the causative drug can result in increase in number of FDE lesions, ultimately developing TEN. Our patient was continued on amitryptilline considering it to be an innocuous drug.
One of our patients developed SJS-TEN to antituberculous treatment (ATT), ATT was stopped and the patient was managed with I/V Ig and steroids. He responded to this management and was referred to the chest physician for further treatment of his pulmonary tuberculosis. He was treated with second line ATT regimen comprising of ethionamide, kanamycin, cycloserin, levofloxacin and para aminosalicylic acid. The patient developed a recurrence of SJS in a more severe form within a week, which was again managed with withdrawal of the drugs, systemic steroids and I/V Ig. As our patient manifested reaction to both these regimens and as INH and ethionamide are structurally related after subsidence of reaction he was restarted on first line ATT, with INH being substituted by levofloxacin and he completed the course of treatment without any further complications.
In previous studies commonest reaction pattern detected was maculopapular rash,[7,9,11,12] or fixed drug eruption.[6] Our study was conducted in patients who required inpatient management in a tertiary care hospital. This could be the reason for the predominance of SCAR in our study. Again this could be the reason for anticonvulsants (known to produce more severe adverse reactions)[7] being the commonest offender in this study as against antimicrobials.[6,9,11]
Eosinophilia was almost an exclusive finding of severe drug reactions, a similar observation was made by Pudukadan et al.[6]
Better prognosis observed in DRESS could be attributed to the milder skin and mucosal involvement in this reaction pattern. Our finding of relatively less severe mucocutaneous involvement in DRESS was noted by previous authors and it is proposed to be due to the expansion of regulatory T (Treg) cells in DRESS that migrate to skin and suppress the activation of effector T-cells. In SJS-TEN spectrum, it is suggested that the migration of Treg cells to skin is inhibited, thus removing their inhibitory role on T-cell inflammatory response.[13] Our observation of flare up noticed in two patients with DRESS, on tapering steroids has been well documented in literature.[14,15] These patients had more severe manifestations including marked derangement of liver enzymes and pneumonitis. The causative drugs were dapsone and carbamazepine respectively.
Though it is suggested that coexisting collagen vascular disorders and genetic factors can predispose to adverse drug reactions, none among the study population had coexisting collagen vascular disease or a family history of drug reaction.
Prolonged hospital stay in SJS-TEN was expected as all these patients had varying degrees of epidermal detachment. The same could be the reason for development of septicemia in 6 SJS-TEN cases. Though not common, ARDS has been described in SJS-TEN, as witnessed in one of our patients, which if not tackled promptly, can end up fatally. Tracheobronchial sloughing and septicemia are the common precipitating causes for ARDS in SJS-TEN. Hence tachypnoea and dyspnoea in CADR, especially SJS-TEN, should be considered as warning signs and should be dealt on an emergency basis. The mortality rate observed among TEN cases in this study was much lower than the previous reports,[12] probably due to early intervention in a referral centre.
Conclusion
The commonest reaction pattern observed in our study was SJS – TEN spectrum of disease. There was a male preponderance and the commonest age group affected was 46-60 years. Eosinophilia was a feature of more severe reactions. Early diagnosis and prompt withdrawl of the offending drug and administration of systemic steroids with or without I/V Ig can be life saving in CADR. Though DRESS was more commonly associated with constitutional symptoms and systemic involvement, more complications and prolonged morbidity were associated with SJS-TEN, probably due to widespread epidermal detachment and due to higher chance of life threatening complications like septicemia and ARDS. We need more studies on drug reactions as the common offending drugs varies with the general prescription practices in a region, which depend on the common infections and the life style diseases prevalent in that particular population.
What is new?
Early diagnosis, prompt withdrawal of the suspected drug and administration of systemic steroids with careful monitoring of vital signs for early detection of complications and management of the same in an ICU with modern critical care facilities can improve the prognosis in SCAR.
Acknowledgment
We are grateful to all the faculty and postgraduates in the department for their invaluable help in conducting this study.
Appendix: Severe cutaneous adverse drug reactions: A clinicoepidemiological study
Case No:
Name: Age: yrs. Sex: M/F
Occupation: Address:
Presenting complaints:
Date of Admission:
Date of onset of illness:
Date of onset of Upper Respiratory Illness (if present)
Date of onset of rash:
Constitutional symptoms (If present):
Conjunctival congestion, Sore throat, Fever, Myalgia, Arthralgia, Cough, Diarrhoea, Abdominal pain, Yellowish discolouration of urine, Pain on micturition
Distribution of rash: Face/Chest/Abdomen/Back of trunk/Upper arms/Fore arms/Thigh/Leg/Palms/Soles
Site of onset of rash: Face/Chest/Abdomen/Back of trunk/Upper arms/Fore arms/Thigh/Leg/Palms/Soles
Rash: Pruritic/painful/asymptomatic/burning sensation
Evolution of disease:
Mucosal involvement: oral/conjunctival/Nasal/Genital
Time interval between constitutional symptoms and mucosal involvement
Mention the offending drug:
Drug intake: Before appearance of constitutional symptoms
Following appearance of constitutional symptoms
Time interval between drug intake and appearance of symptoms:
Past History:
Current medication:
History of drug reaction in the past:
If yes, mention the drug
Specify the type of reaction
History of collagen vascular disease:
If yes, specify the disease
Personal history including addictions:
Family history:
History of drug reactions in family members:
Family history of collagen vascular diseases: Yes/No
If yes, specify the disease:
General examination
Built and nourishment
Pallor/clubbing/cyanosis/jaundice/pedal oedema/lymph node enlargement
Plus Rate: Blood pressure: Respiratory rate: Temperature:
Dermatological examination
Dermatological manifestations:
Morphology of rash:
Sites affected: Face/chest/abdomen/back of trunk/upper arms/forearms/thighs/legs/palms/soles
Mucosal lesions: Conjunctival congestion/Hemorrhagic crusting of lips/Erosions of buccal mucosa/Gingivitis/Erosions of tongue/Nasal erosions/Genital erosions/others
Scalp: Nails:
Examination of systems
Cardio Vascular system:
Central Nervous system:
Respiratory system:
Gastro-intestinal system: Hepatomegaly/Splenomegaly
Lab investigations
Blood R/E
Hb: Tc: Dc: ESR:
Platelet count Absolute eosinophil count Peripheral smear
Urine R/E
Alb: Sugar: M/E
LFT: RFT: Serum Electrolytes:
HBsAg Anti HCV HIV Screening ANA
Chest X-ray PA view Ultrasound abdomen
Final diagnosis
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Roujeau JC, Allanore L, Liss Y, Mockenhaupt M. Severe Cutaneous Adverse Reactions to Drugs (SCAR): Definitions, diagnostic criteria, genetic predisposition. Dermatol Sinica. 2009;27:203–9. [Google Scholar]
- 2.Bilimoria FE, Shah BJ. 3rd ed. Vol. 2. Mumbai: Bhalani Publishing House; 2008. Drug Reactions. IADVL Text Book of Dermatology; pp. 1633–86. [Google Scholar]
- 3.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 4.Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice study II. N Eng J Med. 1991;324:377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 5.Hafner JW, Belknap SM, Squillante MD, Bucheit KA. Adverse drug events in emergency department patients. Ann Emerg Med. 2002;39:258–67. doi: 10.1067/mem.2002.121401. [DOI] [PubMed] [Google Scholar]
- 6.Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care centre in South India. Indian J Dermatol Venereol Leprol. 2004;70:20–4. [PubMed] [Google Scholar]
- 7.Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical Pattern and Causative Agents – A 6 year series from Chandigarh, India. J Postgrad Med. 2001;47:95–9. [PubMed] [Google Scholar]
- 8.Mehta TK, Marquis L, Shetty JN. A study of 70 cases of drug eruptions. Indian J Dermatol Venereol Leprol. 1971;37:2–5. [PubMed] [Google Scholar]
- 9.Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? A 5-year analysis. Br J Dermatol. 2012;166:107–14. doi: 10.1111/j.1365-2133.2011.10623.x. [DOI] [PubMed] [Google Scholar]
- 10.Breathnach SM. Drug reactions. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Vol. 75. UK: Wiley-Blackwell; 2010. pp. 1–177. [Google Scholar]
- 11.Nandha R, Gupta A, Hashmi A. Cutaneous adverse drug reactions in a tertiary care teaching hospital: A North Indian perspective. Int J Appl Basic Med Res. 2011;1:50–3. doi: 10.4103/2229-516X.81982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol. 2008;53:2–8. doi: 10.4103/0019-5154.39732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiohara T, Kano Y, Takahashi R. Current concepts on the diagnosis and pathogenesis of drug-induced hypersensitivity syndrome. Japan Medical Association Journal. 2009;52:347–52. [Google Scholar]
- 14.Gentile I, Talamo M, Borgia G. Is the drug-induced hypersensitivity syndrome (DIHS) due to human herpes virus 6 infection or to allergy-mediated viral reactivation? Report of a case and literature review. BMC Infect Dis. 2010;10:49. doi: 10.1186/1471-2334-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Criado PR, Avancini J, Santi CG, Medrado AT, Rodrigues CE, de Carvalho JF. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A complex interaction of drug, viruses and the immune system. Isr Med Assoc J. 2012;14:577–82. [PubMed] [Google Scholar]


