Abstract
Purpose
Tissue oxygen (O2) levels are among the most important and most quantifiable stimuli to which cells and tissues respond through inducible signaling pathways. Tumor O2 levels are major determinants of the response to cancer therapy. Developing more accurate measurements and images of tissue O2 partial pressure (pO2), assumes enormous practical, biological, and medical importance.
Methods
We present a fundamentally new technique to image pO2 in tumors and tissues with pulse electron paramagnetic resonance (EPR) imaging enabled by an injected, nontoxic, triaryl methyl (trityl) spin probe whose unpaired electron’s slow relaxation rates report the tissue pO2. Heretofore, virtually all in vivo EPR O2 imaging measures pO2 with the transverse electron spin relaxation rate, R2e, which is susceptible to the self-relaxation confounding O2 sensitivity.
Results
We found that the trityl electron longitudinal relaxation rate, R1e, is an order of magnitude less sensitive to confounding self-relaxation. R1e imaging has greater accuracy and brings EPR O2 images to an absolute pO2 image, within uncertainties.
Conclusion
R1e imaging more accurately determines oxygenation of cancer and normal tissue in animal models than has been available. It will enable enhanced, rapid, noninvasive O2 images for understanding oxygen biology and the relationship of oxygenation patterns to therapy outcome in living animal systems.
Keywords: Oxygen, Imaging, EPR, Pulse, R1, Spin Lattice Relaxation, In Vivo, Tumor
Introduction
Importance of EPR O2 Images
Molecular oxygen, O2, is a crucial molecular determinant of states of human health and disease. One third of human deaths are due to diseases of O2 deprivation such as ischemic heart disease and cerebrovascular disease (1). Regions of low O2, hypoxia, thought to be a universal characteristic of solid tumors, (2) reduce the efficacy of radiation therapy in their treatment (3). The importance of organismal response to hypoxia is reflected in the hundreds of genes regulated by hypoxia inducible factor-1 (HIF-1). (4,5) As will be seen in animal model tumor images presented in this paper, large pO2 gradients separate regions of high and low pO2 throughout a tumor. In vivo quantification of tissue pO2 in disease states, thus, requires imaging to fully characterize the oxygenation state of the entire tumor. EPR O2 images may define the mechanism by which hypoxia creates resistance to radiation, which remains elusive. (2) pO2 EPR images registered with either stereotactic biopsies or gene induction images can define quantitative gene induction response to local pO2 - in vivo - in states of health and disease (6).
R1 images solve confounding self-relaxation of O2 EPR Images
Heretofore, in in vivo EPR O2 images, pO2 has been measured via the broadening of the EPR spectral lines of an injected spin probe predominantly through Heisenberg spin exchange (HSE) with local O2. (7) The spin probe is a paramagnetic trityl molecule (8) with a single narrow EPR line whose relaxation times are long enough to enable pulse imaging. Spin packet spectral line width (LW) broadening in a continuous wave (CW) experiment is physically equivalent to increasing the transverse magnetization relaxation rate (R2e) in a pulse experiment. LW=(γeT2e)−1=R2e/γe; γe ≡ gyromagnetic ratio of the electron (9). Both R2e and R1e depend linearly on pO2 (8,10,11). The spin probe collision rate with extremely rapidly relaxing O2, increases R2e, destroying the magnetization phase coherence. EPR images of R2e yielded quantitative O2 images in vivo (12–15) providing about 1 mm resolution, and a method less susceptible to confounding biologic variation than other techniques (2).
HSE between the unpaired electrons of O2 and spin probe similarly increases the spin probe electron spin-lattice or longitudinal relaxation rate R1e, which measures the loss of the trityl spin magnetization energy to the lattice, predominantly to the O2 (7). However, HSE self-relaxation processes, HSE interactions between spin probes, affect R1e and R2e differently. HSE between two trityl molecules with differing EPR frequencies produces additional precession phase shifts that increase the R2e (7). HSE does not alter the total energy of an interacting spin probe pair, and therefore does not affect the electron spin system energy - the longitudinal magnetization. Use of image pulse sequences dependent on R1e reduces probe self-relaxation by nearly an order of magnitude. At this level, essentially the only physiologic R1e relaxation rate increase is due to O2.
Imaging of trityl concentration is difficult
Trityl concentration, referred to as [trityl], is related to the amplitude of the trityl signal in each voxel and might be useful to correct the effect of [trityl] self-relaxation on R2e. However the accuracy in determining [trityl], using local signal amplitude is complicated by the extracellular fluid compartment distribution of trityl (16) and the variability of the extracellular volume fraction in the chaotic tumor environment. Trityl distribution volume varies from voxel to voxel making [trityl] correction difficult, blurring R2e-based pO2 accuracy. We find, here, R1e pO2 images are far less [trityl] dependent and more accurate than R2e -based images.
Imaging longitudinal relaxation rate R1e is straightforward and accurate with pulse EPR
In saline solutions and very low [trityl], R1e is close to R2e at physiologic temperatures. Although quantitative oxygen R1e EPR measurements (11,17) and images (18) have been reported these were continuous wave studies. Pulse imaging is more efficient, shows better precision and, as we will show in this paper, accuracy. (19) This work is the first to demonstrate direct, quantitative, pulse in vivo R1e images. The long electron relaxation times of the trityl OX063 (8) with T1e and T2e of ~ 7 µs at hypoxic conditions, and consequent slow relaxation rates enable pulse R1e images with current RF technology. We read out R1e from each voxel using inversion recovery (IR) (20), imaging the recovering longitudinal magnetization as a function of time after the inversion pulse using electron spin echo (ESE) imaging (14,21) - a tomographic frequency-encoding method. We thus refer to inversion recovery with ESE readout as IRESE.
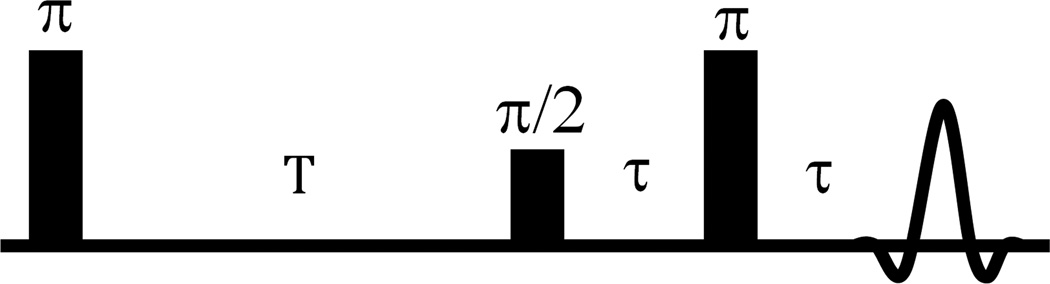
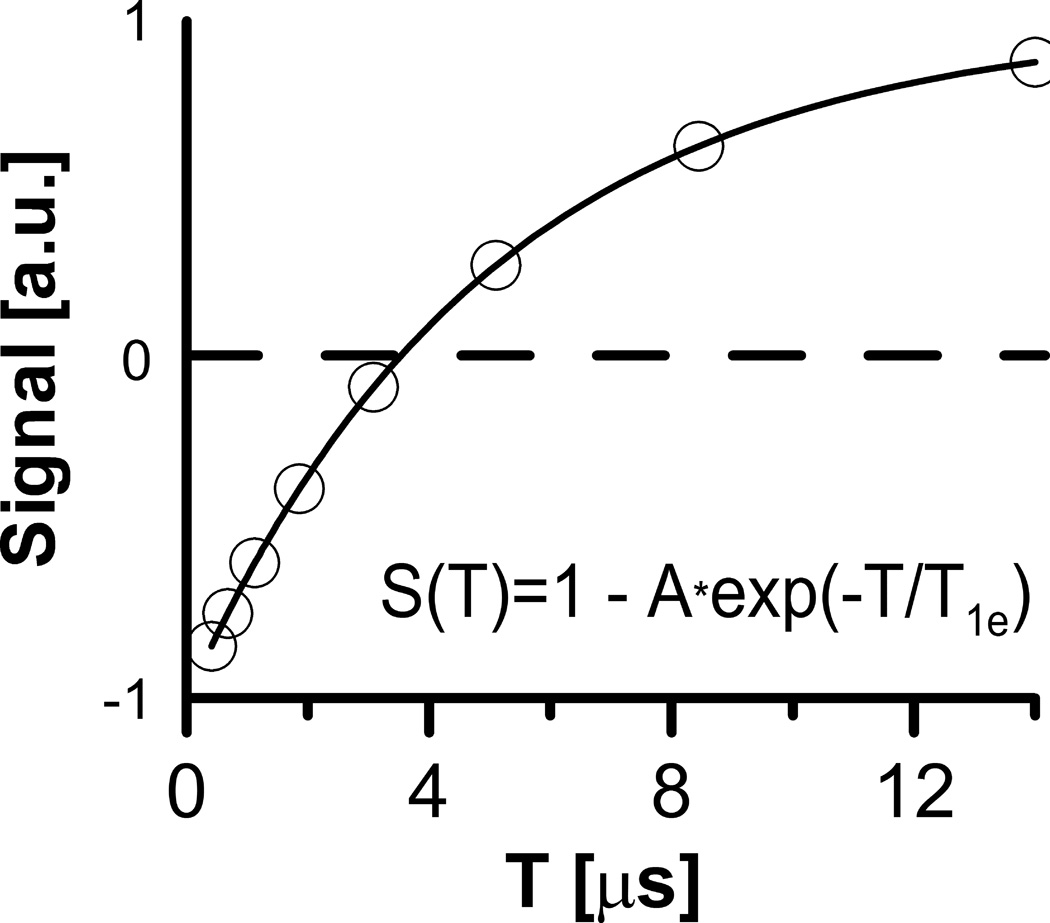
The inversion recovery sequence IRESE (Fig. 1), inverts the spin polarization with a π–pulse preceding the ESE π/2–π detection sequence. The recovery is measured as a function of the delay T (Fig. 2) between the inversion pulse and the ESE detection sequence. The coefficient A in the equation in Fig. 2 is less than or equal to, but near, two. Values less than 2 account for incomplete EPR line inversion. A single echo time τ is fixed at an optimum slightly longer than the imager dead time.
Figure 1.
IRESE pulse sequence for R1e imaging.
Figure 2.
Simulation of a single voxel’s signal amplitude dependence on T in IRESE sequence. T1e = 5 µs was used.
This study compares R1e with R2e imaging using a 250 MHz pulse imager (14). We demonstrate that in vivo R1e imaging shows much higher absolute pO2 accuracy, overcoming the limitations imposed by [trityl] relaxation on R2e imaging.
Methods
Imaging Methods
A pulse 250 MHz imager (14) was enhanced with a passive transmit-receive switch (22) and π/2- and π- pulses of equal duration/bandwidth (23). The imager was controlled with SpecMan4EPR v. 1.1.6 (24).
Image acquisition time and spatial resolution were fixed at, respectively, 10 minutes and 1.5 mm. Spatial resolution of the ESE and IRESE images were defined by the Rayleigh resolution criterion (14,25). We used the same pulse sequences for phantoms and for animal imaging. The standard deviation of the relaxation times/rates in homogeneous phantoms, excluding two outer layers to avoid partial volume artifacts, was used as an estimation of relaxation time/rate errors. Tables 1 and 2 present parameters of the R1e and R2e sequences. The repetition time, TR, for ESE sequences was adjusted to keep constant the delay between the last pulse in a sequence and the first pulse of the next sequence. For accurate R1e in IRESE images the image recorded at infinite recovery time T was equated to an image recorded without an inversion pulse. For voxel intensity fitting to an exponential recovery function, a T equal to 36 µs was assigned to this image.
Table 1.
Pulse sequences and imaging protocols.
| Protocol | Description |
|---|---|
| Non-imaging two-pulse ESE | π/2-τ-π-τ-echo; 35 ns π/2 and π RF pulses; 80 τ's logarithmically spaced between 630 ns and 14 µs; 16-step phase cycling; 70 µs repetition time; echo is integrated; τs are measured in random order. |
| Non-imaging IRESE | π-T-π/2-τ-π-τ-echo; 35 ns π/2 and π RF pulses; τ = 630 ns; 16-step phase cycling for detection sequence; 80 Ts are spaced logarithmically between 0.5 µs and 32 µs; 80 µs repetition time; echo is integrated; Ts are measured in random order. |
| Two-pulse ESE imaging | π/2-τ-π-τ-echo; 35 ns π/2 and π RF pulses; time trace 1500 points with 4 ns dwell time; 16-step phase cycling, 37472 acquisitions per τ, including phase cycling; 5 τs are spaced logarithmically between 0.63 µs and 2.4 µs; TLFR = 10.37 µs; |G|=15 mT/m; imaging time 10 minutes. |
| IRESE imaging | π-T-π/2-τ-π-τ-echo; 35 ns π/2 and π RF pulses; time trace 1500 points with 4 ns dwell time; τ = 630 ns; 16-step phase cycling applied only for detection sequence, 9600 acquisitions per T, including phase cycling; 8 Ts are spaced logarithmically between 0.41 µs and 14 µs; TLFR = 25 µs; |G|=15 mT/m; imaging time 10 minutes. |
Table 2.
Parameters of pulse sequences
| Pulse length | RF power [W] |
Bandwidth [MHz] |
Transmitted Average Power [W] |
|
|---|---|---|---|---|
| 2pESE (T2e) | 35 ns, π/2 and π | 39.6 (π/2), 158.5 (π) | 8.7 | 0.57 |
| IRESE (T1e) | 35 ns, π/2 and π | 39.6 (π/2), 158.5 (π) | 8.7 | 0.42 |
For images, (14,26) 208 equal solid angle projections (27) were acquired; gradient |G⃗|=15 mT/m; field of view =4.24 cm. A 53 baseline projections obtained with 1.5 mT lower main field were acquired with every fourth projection and subtracted from the previous four projections. Projection numbers were expanded with four-fold cubic B-spline interpolation (27) and were filtered with a 3D Ram-Lak filter cutoff at one half the Nyquist frequency. Voxels with amplitude less than 15% maximum at the shortest delay were eliminated (thresholded). (14).
The system frequency band-pass function for each acquisition technique was measured using zero gradient sample signal amplitude at 50 spanning B0 fields (14). Projections were normalized using this function. Image analysis was performed with in-house software written using MATLAB (Mathworks, Inc., Natick, MA).
The spatial resolution of an image can be quantified by the response of an image to an abrupt step function change in sample density fitted with the Gauss error function (erf(x/2σ)). The width of this error function σ=1.5mm is an estimate of the ESE image spatial resolution. IRESE image has the same spatial resolution.
Spin Probe
Spin probe was the trityl OX063 radical methyl-tris[8-carboxy-2,2,6,6-tetrakis[2-hydroxyethyl]benzo[1,2-d:4,5-d’]bis[1,3]dithiol-4-yl]-trisodium salt, GE Healthcare (LittleChalfont, Buckinghamshire, UK). Phantoms with 1 mM spin probe in saline were glass cylinders 9.5 mm i.d., 45 mm long. The 0% O2 sample was degassed via freeze-pump-thaw; the 9.3% O2 sample was bubbled with a 9.3%:90.7% oxygen-nitrogen mixture and epoxy sealed. For measuring oxygen concentration dependence, the phantoms were bubbled in situ with humidified gas mixture. 30 minutes or more were given for oxygen equilibration during these measurements.
Non-imaging vs. imaging conditions
Acquisition of spatial information requires considerable time, which, for in vivo imaging, is limited by the animal physiology. Therefore imaging protocols have to balance the precision of the measurements with the experiment’s duration. As a result, the relaxation times in imaging protocols are estimated from only five points (R2e) or eight points (R1e) on the decay curve. Such restrictions do not apply for non-imaging measurements on phantoms, which have larger numbers of delays (T) from the inversion pulse (80 vs 5 R2e – 8 R1e for imaging) and wider TR interval (at least 5 hypoxic T1e for non-imaging vs ~ T1e for imaging). Non-imaging measurements were repeated ~50 times, 20 seconds for each measurement; data were fitted independently, and the average was presented.
Animal Imaging
FSa fibrosarcomas were grown on the legs of 6–8-week-old C3H mice (HSD, Indianapolis, IN) immobilized with a partial circumference vinyl polysiloxane cast (GC Dental Products, Kasugai, Japan) (28). For Fig. 4, OX063 was injected IV 0.56 mmol/kg followed by infusion at 0.63 mmol/kg/hr. In Fig. 5, after each R1e/R2e image pair additional 0.21 mmol/kg OX063 was injected, and infusion was increased by 0.35 mmol/kg /hour, to a maximum of 3.85 mmol/kg /hour. This was performed on three animals with consistent results. Tumor was defined by T2 enhancement in RARE MRI registered with EPR images (29).
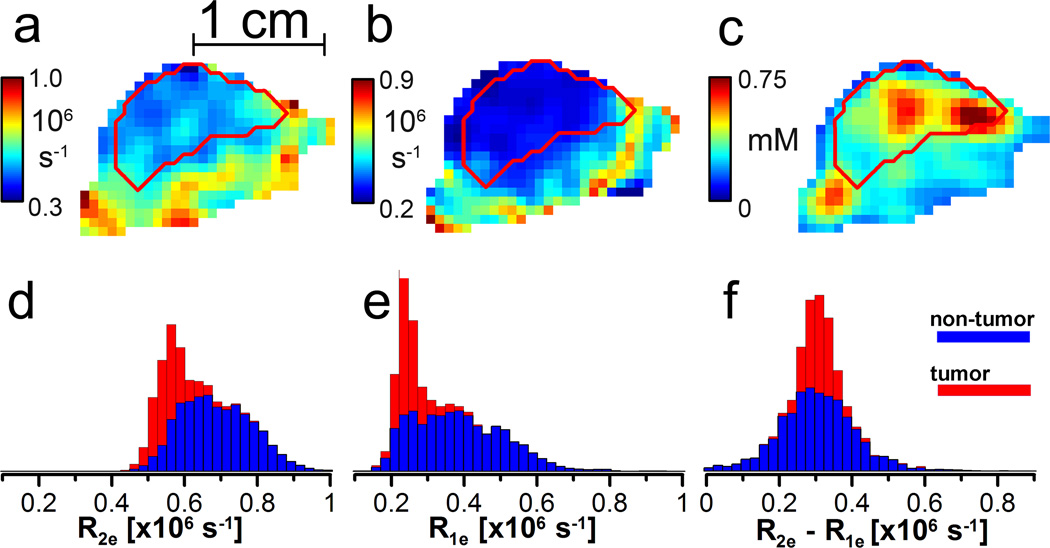
Figure 4.
a–c: sagittal slice (0.7 mm) of a mouse leg bearing a tumor. a. R2e image from ESE; b. R1e image from IRESE; and c. [trityl] image from ESE. The tumor contour is obtained from a registered MRI. d–f: stacked histograms of d. R2e; e. R1e; and f. the difference between R1e and R2e.
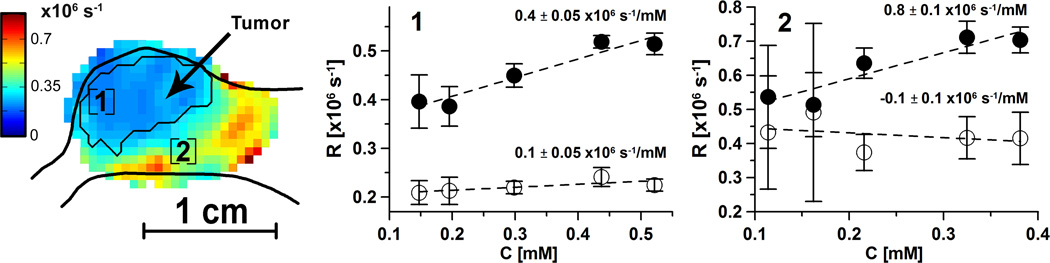
Figure 5.
R1e (◦) and R2e (•) in a mouse vs [trityl] as spin probe is infused at different rates. [trityl] is obtained by normalization of animal signal intensity in each voxel on the voxel intensity of phantom with 1mM concentration. A sagittal slice of R1e image of the tumor-bearing leg is shown on the left. Leg profile and tumor contour are obtained from registered T2-weighted MRI. R1e and R2e [trityl] dependences for two areas [1] and [2] obtained by averaging voxels in ~8 mm3 cube (27 voxels) are shown. Error bars are the R1e and R2e standard deviations. Area 1 is located in the tumor, and area 2 is in the muscle. The slopes of [trityl] dependence and 50% confidence intervals are given in the plots.
Animal experiments followed USPHS policy, and were approved by the Institutional Animal Care and Use Committee.
Results
In vitro studies show reduction of R1e sensitivity to confounding [trityl] relaxation by nearly an order of magnitude relative to that of R2e
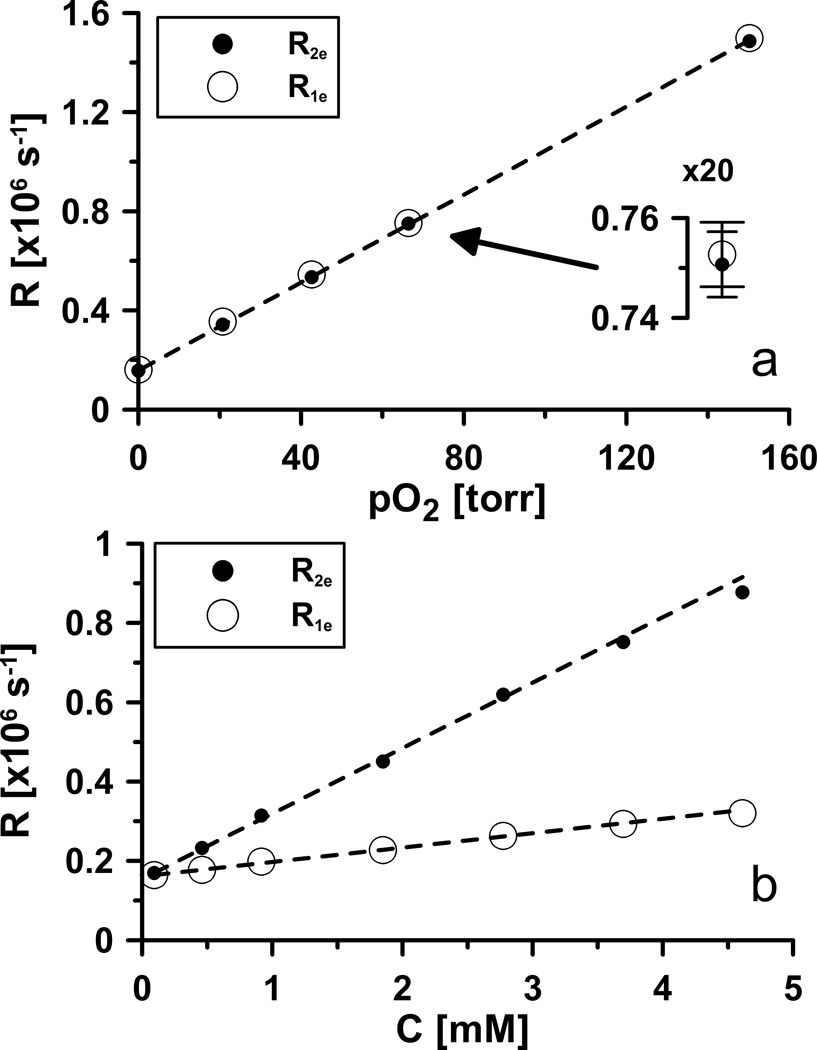
We performed studies in vitro by using the OX063 spin probe, as described in Methods (15,30,31). Sodium chloride concentration affects the relaxation times (8). Thus, normal saline (145 mM NaCl) resembling animal condition, was used as solvent (1). Temperature, was kept at 37°C, as in rodents and humans (32). These conditions affected the relaxation times and made them substantially different from the measurements on the same spin probe dissolved in water and performed at room temperature; see for example (33). In saline sodium ions allow the tri-acid OX063, whose charge is −3 at physiologic pH, to approach more closely to each other than in water, increasing [trityl] dependent transverse self-relaxation. The dependence of OX063 relaxation rates on pO2 is given in Figure 3a. The experimental data were corrected for the effect of spin probe concentration, referred to as [trityl] by applying linear relation between [trityl] and relaxation rate (Fig. 3b). It should be noted that relaxation rates of spin probe in water show weaker and non-linear dependence on [trityl] (data not shown). The reasons for that are under investigation. There was no significant difference between the slopes of the linear dependences of R1e and R2e on pO2. However, there was a dramatic difference in the slopes of the dependences of R1e and R2e on spin probe concentration, referred to as [trityl] (Fig. 3b). R2e vs [trityl] had nearly five times greater slope than did R1e.
Figure 3.
Relaxation rates of OX063 dissolved in saline at 37°C.
a. [trityl] corrected dependence (concentration independent) of relaxation rates on pO2. The data were obtained using 0.46 mM sample. The relaxation rates were then extrapolated to zero [trityl] by subtracting 0.46mM·0.165·106 s−1/mM =76·103 s−1 for R2e, and 0.46mM·36.3·103 s−1/mM =17·103 s−1 for R1e. Best fit: R1e = 8.9·103 s−1/torr * pO2 +1.6·105 s−1; R2e = 8.9·103 s−1/torr * pO2 + 1.6·105 s−1.
b. [trityl] dependences of relaxation rates R1e and R2e. Best fit: R1e = 36.3·103 s−1/mM * [trityl] +0.16·105 s−1; R2e = 0.165·106 s−1/mM [trityl] + 0.16·105 s−1.
The 95% confidence intervals for fit parameters are: ±1·104 s−1 for offsets, ±1.2·102 s−1/torr for O2 and ±7·103 s−1/mM for concentration proportionality coefficients.
The quantitative dependence of R2e on [trityl] is much weaker than on pO2 or O2 concentration, referred to as [O2]. At 37 °C, [O2] in saline solution is 219 µM at 159 torr or ~1.4 µM/torr. Thus, the sensitivity of trityl R2e to [O2] is ~6.3·106 s−1/mM, but only 0.165·106 s−1/mM for [trityl].
The results of R1e imaging and relaxation measurements of phantoms with 0% and 9.3% O2 (71 torr pO2 at 37 °C and atmospheric pressure) are summarized in Table 3. These [O2] bracket relevant values from pO2 studies of hypoxia in animals (15,34). The relaxation times determined under non-imaging conditions are given in the footnotes of the table. No significant difference between average relaxation times determined under imaging and non-imaging conditions was found.
Table 3.
Precision of images estimated as a standard deviation of relaxation times in an image of homogeneous phantom. Images are obtained in 10 minutes on phantom containing 1 mM OX063 dissolved in normal saline at 37°C. No [trityl] correction is applied.
| 0% pO2 | 9.3% pO2 | |||||
|---|---|---|---|---|---|---|
| Pulse Sequence |
Average T2e or T1e [µs] |
Standard deviation of T2e or T1e [µs] |
Standard deviation of R2e or R1e [x 103 s−1] |
Average T2e or T1e [µs] |
Standard deviation of T2e or T1e [µs] |
Standard deviation of R2e or R1e [×103 s−1] |
| TR measurements (T2e and R2e) | ||||||
| 2pESE | 3.1 | 0.2 | 7.9* | 1.25 | 0.07 | 46* |
| SLR measurements (T1e and R1e) | ||||||
| IRESE | 5.0 | 0.3 | 7.9* | 1.31 | 0.15 | 90* |
Non-imaging relaxation times: for 0% O2 sample T2e = 2.99 µs (two-pulse ESE), T1e = 4.82 µs (IRESE); for 9.3% O2 sample T2e = 1.24 µs (two-pulse ESE), T1e = 1.33 µs (IRESE).
7.9·103 s−1 corresponds to 0.9 torr; 46·103 s−1 corresponds to 5 torr; 90·103 s−1 corresponds to 10 torr.
Residual dependence of R1e on [trityl] is likely due to spectral diffusion, the interaction of excited spins with the other spins whose EPR frequencies are beyond the excitation bandwidth (20). Such spins may be from trityl molecules containing 13C (1.109% natural abundance) producing hyperfine lines split up to 12 G from the central line, (35) considerably beyond the excitation bandwidth of the pulse sequence. The results of R2e measurements using ESE are given in the table for comparison. To estimate errors, the standard deviation of the relaxation times was estimated for homogeneous phantom voxels, excluding regions with edge artifacts. The R2e image had a standard deviation smaller than that of the R1e imaging method. However, for 0% O2 the standard deviation of the R1e image approached that of the R2e image.
R1e in the tumor of a live animal shows far more freedom from [trityl] relaxation
For a demonstration of the robustness of R1e imaging on a live animal tumor (Fig. 4b), we compared the IRESE image slice with the same slice from the R2e image (Fig. 4a), obtained from a two-pulse ESE image on the same animal 10 minutes later. The brighter, richly colored areas in both of the images are well oxygenated, whereas the darker, more intensely blue areas are hypoxic. The tumor outline from a registered T2-weighted MRI is shown in both the images as red contours. The outlines and general oxygenation patterns are very similar. However, absolute voxel pO2 values differ considerably. Figure 4c presents the [trityl] image obtained with two-pulse ESE. Figures 4d and 4e are histograms of the R2e and R1e relaxation rates, respectively. The red-colored histograms are from the voxels enclosed by the red tumor contours in Figs. 4a–c. The blue histogram bars are from the leg tissue outside the tumor contours. They are added to the tumor histogram bars so that the ultimate height of the blue plus red histogram bars represents the total number of image voxels with the indicated relaxation rate.
The mode of the overall R1e distribution from the tumor shown in Fig. 4e is nearly 0.35·106 s−1 smaller than that from the R2e image. In the distributions of the R1e and R2e, there are two components with different modes and widths – the tumor and the residual leg area. The sharper component with the lower mode, localized primarily in the tumor area, is more clearly distinguished in the R1e image. That component is associated with the narrower distribution of relaxation rates expected in the hypoxic tumor area and shows the improved performance of R1e imaging. The slowest R1e observed in the animal experiments (Fig. 4e) are very close to the rates found in deoxygenated phantoms. This is expected because tumors are frequently hypoxic. R2e rates, however, are ~0.35·106 s−1 higher than R1e rates (Fig. 4f). Because the relaxation rates due to different mechanisms are additive, the shift of the distribution toward higher values indicates that R2e images are more susceptible to O2-independent relaxation than is R1e. At room temperature and at a negligible [trityl] the R2e and R1e are similar.
Ultimately, the advantage of R1e-based pO2 imaging over R2e-based pO2 imaging lies in its reduced susceptibility to confounding variation from [trityl]-induced relaxation. We demonstrated this by artificially increasing [trityl] by increasing the rate of trityl infusion to an animal and assessing the changes in the relaxation rates in selected regions. Although the local tissue oxygenation in subvolumes of tumors is known to vary (36,37), we assumed the average tissue oxygenation during the experiment to be constant. We justify this assumption below based on the results. In the absence of changes in oxygenation all changes in the relaxation rates should be from [trityl] relaxation effect. As a surrogate for the local [trityl], we used the voxel signal intensity obtained from R2e images normalized to the intensity of voxels in a 1 mM phantom. The voxel signal intensity is R2e-independent because the spin echo amplitude was extrapolated to τ=0. In Figure 5, the change in relaxation rates was imaged by alternating ESE and IRESE images. The [trityl], estimated from the ESE images, in animal tissues was stabilized prior to imaging by comparison of consecutive image intensities. Two regions of the image, one directly in the tumor volume and the other just outside the tumor volume in a well-perfused region, were compared for each image type. The regions consisted of a cube of 3×3×3=27 voxels, as seen in the leftmost image which shows a sagittal slice including the regions. The R2e and R1e are plotted to the right of the image slice from each region. The [trityl] dependence coefficients of R2e and R1e are indicated along with their uncertainties determined from the scatter of values about the regression lines. The R2e values, for each location, depend strongly on the apparent [trityl] while the R1e values are independent of [trityl] to within the value uncertainties shown. The effect of [trityl] on R2e is considerably stronger than the effect of cycling hypoxia (36,37) and, more importantly is observed not only in tumor but in all anatomic areas.
Discussion
This work demonstrates that R1e images have nearly an order of magnitude reduced sensitivity to self-relaxation in vivo and, thus, higher accuracy. The precision of R1e and R2e images is very similar, especially at low [O2]. Figure 5 shows that R2e does indeed depend on [trityl] in vivo. The trend is clearly visible because the range of [trityl] in Figure 5b is far larger than typically found in in vivo images. The slopes of R2e [trityl] dependences in Fig. 5 ranging from 0.2·106 to 0.9·106 s−1/mM are considerably stronger than that found in the phantoms (0.165·106 s−1/mM). In part, this may be due to our underestimation of the real spin probe concentration. The tri-acid spin probe cannot penetrate the cell membrane at physiologic pH (16). Its extracellular distribution volume can be as small as 30% of the tissue volume (38) increasing the actual concentration by a factor of three relative to that based on voxel intensity. The [trityl] dependence of R1e is five times smaller in phantoms and is undetectable in vivo, Fig. 5, areas [1] and [2]. The pO2, inferred from the R1e images in each voxel are, to within the uncertainty, accurate and are the absolute measurements of tissue or tumor oxygenation.
As noted above, virtually all in vivo EPR O2 images have exploited sensitivity of R2e to local pO2, except for one qualitative study (18). The present study demonstrates, for the first time, pulse EPR O2 imaging in live animals that is sensitive to the spin probe electron R1e. The long relaxation times of trityls and the O2-induced changes in R1e are much more favorable than with the nitroxides used in the past as an infusible spin probe for O2 imaging in vivo (39,40). R1e-based O2 imaging is superior to R2e or R*2e imaging modalities because of the reduced sensitivity to [trityl]. The [trityl] in vivo is difficult to control and, given the unknown volume in each voxel occupied by the extracellular trityl spin probe, is difficult to determine accurately. The accuracy of R1e O2 images is thus improved by virtually eliminating sensitivity to trityl concentration.
Conclusions
R1e EPR O2 imaging with trityl spin probes is feasible and has the same precision as R2e imaging. The absolute accuracy of this method is superior to R2e imaging because of the smaller confounding effect of environmental parameters other than O2 on R1e. Therefore, R1e EPR imaging should be considered as a primary method for oxymetry. Ten-minute images of a 1 mM hypoxic sample provide 1 torr pO2 resolution with 1–1.5 mm spatial resolution (14,19).
The improved accuracy of the O2 images, presented here, should further enhance animal studies of tissue and tumor oxygenation. The low radiofrequency at which these studies have been undertaken and the large animal tumors already studied with R2e pO2 images (29) argue for the distinct possibility for measurements in human tumors. pO2 images can direct therapy of cancers with radiation. The enhanced accuracy of R1e pO2 imaging shown here argues strongly for this technique to be incorporated in future animal and perhaps human images.
Acknowledgments
Work was supported by NIH grants P41EB002034 and CA98575.
References
- 1.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18 ed. Vol. 1. New York, NY: McGraw Hill; 2011. p. 3610. [Google Scholar]
- 2.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82(10):699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 3.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8(5):588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107(1):1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 6.Elas M, Hleihel D, Barth ED, Haney CR, Ahn KH, Pelizzari CA, Epel B, Weichselbaum RR, Halpern HJ. Where it's at really matters: in situ in vivo vascular endothelial growth factor spatially correlates with electron paramagnetic resonance pO2 images in tumors of living mice. Mol Imaging Biol. 2011;13(6):1107–1113. doi: 10.1007/s11307-010-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molin YN, Salikhov KM, Zamaraev KI. In: Spin Exchange: Principles and Applications in Chemistry and Biology. Goldanskii VI, Gomer R, Schafer FP, Toennies JP, editors. Berlin: Springer-Verlag; 1980. p. 242. [Google Scholar]
- 8.Ardenkjaer-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, Golman K. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson. 1998;133(1):1–12. doi: 10.1006/jmre.1998.1438. [DOI] [PubMed] [Google Scholar]
- 9.Abragam A. Principles of Nuclear Magnetism. Oxford: Oxford University; 1961. [Google Scholar]
- 10.Popp CA, Hyde JS. Effects of Oxygen on Electron-Paramagnetic-Resonance of Nitroxide Spin-Label Probes of Model Membranes. J Magn Reson. 1981;43(2):249–258. [Google Scholar]
- 11.Subczynski WK, Hyde JS. The Diffusion-Concentration Product of Oxygen in Lipid Bilayers Using the Spin-Label T1 Method. Biochim Biophys Acta. 1981;643(2):283–291. [PubMed] [Google Scholar]
- 12.Halpern HJ, Yu C, Peric M, Barth E, Grdina DJ, Teicher BA. Oxymetry Deep in Tissues with Low-Frequency Electron-Paramagnetic-Resonance. Proc Natl Acad Sci USA. 1994;91(26):13047–13051. doi: 10.1073/pnas.91.26.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian S, Devasahayam N, Murugesan R, Yamada K, Cook J, Taube A, Mitchell JB, Lohman JA, Krishna MC. Single-point (constant-time) imaging in radiofrequency Fourier transform electron paramagnetic resonance. Magn Reson Med. 2002;48(2):370–379. doi: 10.1002/mrm.10199. [DOI] [PubMed] [Google Scholar]
- 14.Epel B, Sundramoorthy SV, Mailer C, Halpern HJ. A versatile high speed 250-MHz pulse imager for biomedical applications. Conc Magn Reson B. 2008;33B(3):163–176. doi: 10.1002/cmr.b.20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elas M, Williams BB, Parasca A, Mailer C, Pelizzari CA, Lewis MA, River JN, Karczmar GS, Barth ED, Halpern HJ. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): Methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magnet Reson Med. 2003;49(4):682–691. doi: 10.1002/mrm.10408. [DOI] [PubMed] [Google Scholar]
- 16.Williams BB, al Hallaq H, Chandramouli GV, Barth ED, Rivers JN, Lewis M, Galtsev VE, Karczmar GS, Halpern HJ. Imaging spin probe distribution in the tumor of a living mouse with 250 MHz EPR: correlation with BOLD MRI. Magn Reson Med. 2002;47(4):634–638. doi: 10.1002/mrm.10089. [DOI] [PubMed] [Google Scholar]
- 17.Kusumi A, Subczynski WK, Hyde JS. Oxygen-Transport Parameter in Membranes as Deduced by Saturation Recovery Measurements of Spin-Lattice Relaxation-Times of Spin Labels. P Natl Acad Sci-Biol. 1982;79(6):1854–1858. doi: 10.1073/pnas.79.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hama Y, Matsumoto KI, Murugesan R, Subramanian S, Devasahayam N, Koscielniak JW, Hyodo F, Cook JA, Mitchell JB, Krishna MC. Continuous wave EPR oximetric imaging at 300 MHz using Radiofrequency power saturation effects. Antioxidants & Redox Signaling. 2007;9(10):1709–1716. doi: 10.1089/ars.2007.1720. [DOI] [PubMed] [Google Scholar]
- 19.Epel B, Sundramoorthy SV, Barth ED, Mailer C, Halpern HJ. Comparison of 250 MHz electron spin echo and continuous wave oxygen EPR imaging methods for in vivo applications. Medical Physics. 2011;38(4):2045–2052. doi: 10.1118/1.3555297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweiger A, Jeschke G. Principles of Pulse Electron Paramagnetic Resonance. Oxford University Press; 2001. [Google Scholar]
- 21.Mailer C, Sundramoorthy SV, Pelizzari CA, Halpern HJ. Spin echo spectroscopic electron paramagnetic resonance imaging. Magnet Reson Med. 2006;55(4):904–912. doi: 10.1002/mrm.20849. [DOI] [PubMed] [Google Scholar]
- 22.Sundramoorthy SV, Epel B, Mailer C, Halpern HJ. A Passive Dual-Circulator Based Transmit/Receive Switch for Use with Reflection Resonators in Pulse Electron Paramagnetic Resonance. Conc Magn Reson B. 2009;35B(3):133–138. doi: 10.1002/cmr.b.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quine RW, Tseytlin M, Eaton SS, Eaton GR. A Very Fast Switched Attenuator Circuit for Microwave and R.F. Applications. Concepts Magn Reson Part B Magn Reson Eng. 2010;37B(2):39–44. doi: 10.1002/cmr.b.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epel B, Gromov I, Stoll S, Schweiger A, Goldfarb D. Spectrometer manager: A versatile control software for pulse EPR spectrometers. Conc Magn Reson B. 2005;26B(1):36–45. [Google Scholar]
- 25.Ahn KH, Halpern HJ. Simulation of 4D spectral-spatial EPR images. J Magn Reson. 2007;187(1):1–9. doi: 10.1016/j.jmr.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn KH, Halpern HJ. Spatially uniform sampling in 4-D EPR spectral-spatial imaging. J Magn Reson. 2007;185(1):152–158. doi: 10.1016/j.jmr.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn KH, Halpern HJ. Comparison of local and global angular interpolation applied to spectral-spatial EPR image reconstruction. Med Phys. 2007;34(3):1047–1052. doi: 10.1118/1.2514090. [DOI] [PubMed] [Google Scholar]
- 28.Haney CR, Fan X, Parasca AD, Karczmar GS, Halpern HJ, Pelizzari CA. Immobilization using dental material casts facilitates accurate serial and multimodality small animal imaging. Conc Magn Reson B. 2008;33B(2):138–144. doi: 10.1002/cmr.b.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epel B, Haney CR, Hleihel D, Wardrip C, Barth ED, Halpern HJ. Electron paramagnetic resonance oxygen imaging of a rabbit tumor using localized spin probe delivery. Medical Physics. 2010;37(6):2553–2559. doi: 10.1118/1.3425787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto K, Subramanian S, Devasahayam N, Aravalluvan T, Murugesan R, Cook JA, Mitchell JB, Krishna MC. Electron paramagnetic resonance imaging of tumor hypoxia: enhanced spatial and temporal resolution for in vivo pO2 determination. Magn Reson Med. 2006;55(5):1157–1163. doi: 10.1002/mrm.20872. [DOI] [PubMed] [Google Scholar]
- 31.Elas M, Ahn KH, Parasca A, Barth ED, Lee D, Haney C, Halpern HJ. Electron paramagnetic resonance oxygen images correlate spatially and quantitatively with oxylite oxygen measurements. Clinical Cancer Research. 2006;12(14):4209–4217. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]
- 32.Krogh A. The Comparative Physiology of Respiratory Mechanisms. Philadelphia: University of Philadelphia Press; 1941. [Google Scholar]
- 33.Owenius R, Eaton GR, Eaton SS. Frequency (250 MHz to 9.2 GHz) and viscosity dependence of electron spin relaxation of triarylmethyl radicals at room temperature. J Magn Reson. 2005;172(1):168–175. doi: 10.1016/j.jmr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Elas M, Bell R, Hleihel D, Barth ED, Mcfaul C, Haney CR, Bielanska J, Pustelny K, Ahn KH, Pelizzari CA, Kocherginsky M, Halpern HJ. Electron paramagnetic resonance oxygen image hypoxic fraction plus radiation dose strongly correlates with tumor cure in FSA fibrosarcomas. International Journal of Radiation Oncology Biology Physics. 2008;71(2):542–549. doi: 10.1016/j.ijrobp.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowman MK, Mailer C, Halpern HJ. The solution conformation of triarylmethyl radicals. J Magn Reson. 2005;172(2):254–267. doi: 10.1016/j.jmr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Yasui H, Matsumoto S, Devasahayam N, Munasinghe JP, Choudhuri R, Saito K, Subramanian S, Mitchell JB, Krishna MC. Low-Field Magnetic Resonance Imaging to Visualize Chronic and Cycling Hypoxia in Tumor-Bearing Mice. Cancer Research. 2010;70(16):6427–6436. doi: 10.1158/0008-5472.CAN-10-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging Cycling Tumor Hypoxia. Cancer Research. 2010;70(24):10019–10023. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganong WF. Review of Medical Physiology. San Mateo, CA: Appleton & Lange; 1987. [Google Scholar]
- 39.Halpern HJ, Yu C, Peric M, Barth E, Grdina DJ, Teicher BA. Oxymetry deep in tissues with low-frequency electron paramagnetic resonance. Proc Natl Acad Sci USA. 1994;91(26):13047–13051. doi: 10.1073/pnas.91.26.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velan SS, Spencer RG, Zweier JL, Kuppusamy P. Electron paramagnetic resonance oxygen mapping (EPROM): direct visualization of oxygen concentration in tissue. Magn Reson Med. 2000;43(6):804–809. doi: 10.1002/1522-2594(200006)43:6<804::aid-mrm5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]