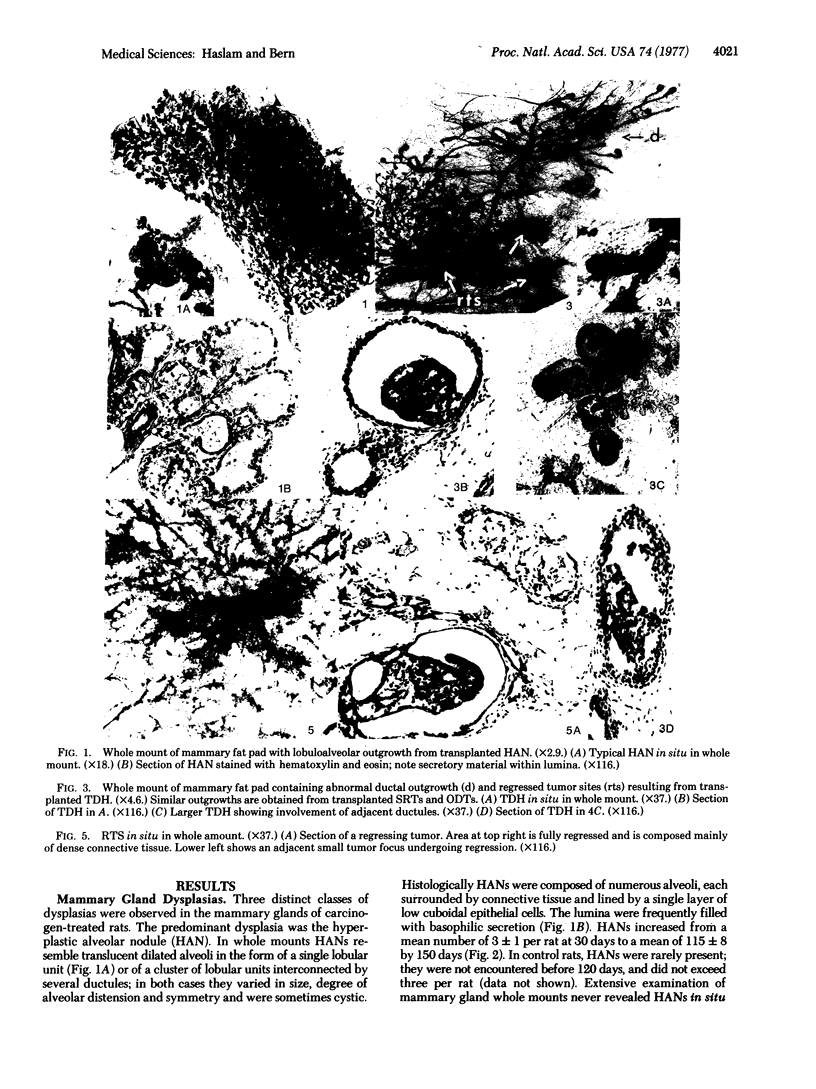
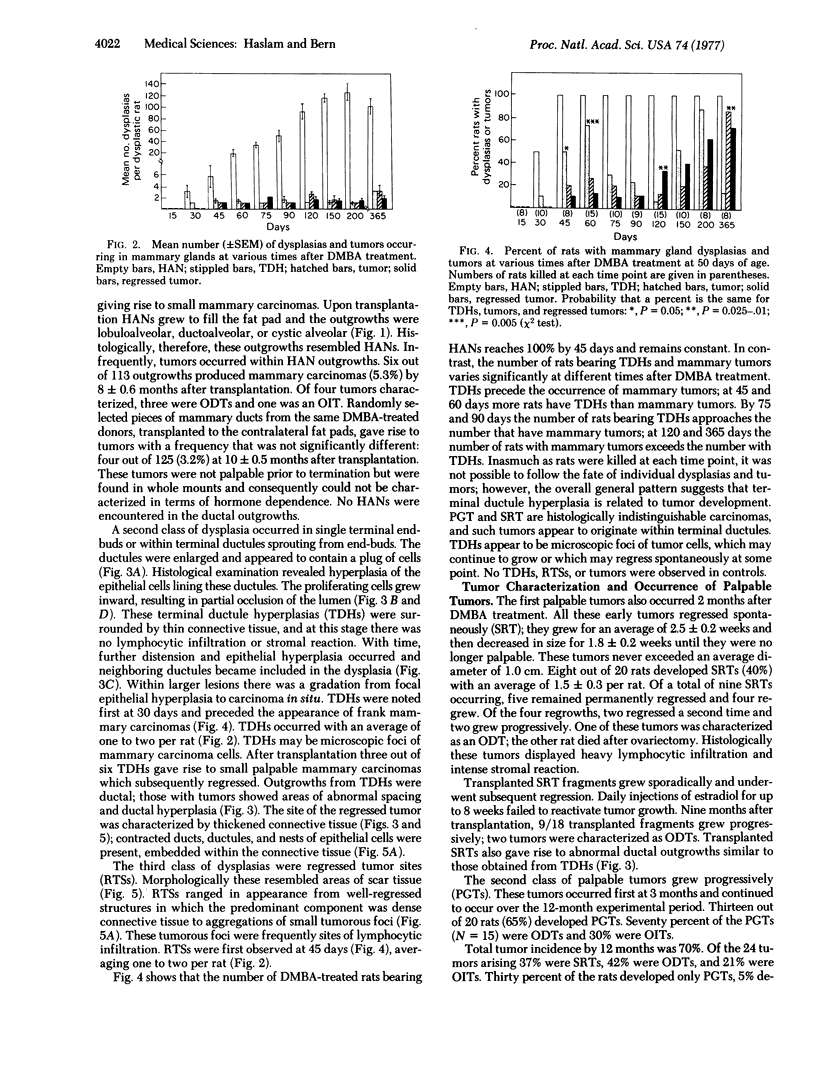
Abstract
The histopathogenesis and growth behavior of mammary tumors and dysplasias induced by a single intragastric dose of 7,12-dimethylbenz[a]anthracene in 50-day-old virgin female Lewis rats were examined both in situ and after transplantation into gland-free mammary fat pads of syngeneic hosts. Terminal mammary ductules are indicated as a site of origin of both ovarian hormone-dependent mammary tumors and spontaneously gegressing mammary tumors, and terminal ductule hyperplasia appears to be an early stage in mammary tumor formation. The precancerous nature of hyperplastic alveolar nodules induced by dimethylbenzanthracene in rats has been further examined, and our studies indicate that these nodules are not significantly preneoplastic.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aidells B. D., Daniel C. W. Hormone-dependent mammary tumors in strain GR-A mice. I. Alternation between ductal and tumorous phases of growth during serial transplantation. J Natl Cancer Inst. 1974 Jun;52(6):1855–1863. doi: 10.1093/jnci/52.6.1855. [DOI] [PubMed] [Google Scholar]
- Beuving L. J., Faulkin L. J., Jr, DeOme K. B., Bergs V. V. Hyperplastic lesions in the mammary glands of Sprague-Dawley rats after 7,12-dimethyl-benz[a]anthracene treatment. J Natl Cancer Inst. 1967 Sep;39(3):423–429. [PubMed] [Google Scholar]
- Beuving L. J. Mammary tumor formation within outgrowths of transplanted hyperplastic nodules from carcinogen-treated rats. J Natl Cancer Inst. 1968 Jun;40(6):1287–1291. [PubMed] [Google Scholar]
- DAO T. L., TANAKA Y., GAWLAK D. TUMOR INDUCTION IN TRANSPLANTED MAMMARY GLANDS IN RATS. J Natl Cancer Inst. 1964 Jun;32:1259–1275. doi: 10.1093/jnci/32.6.1259. [DOI] [PubMed] [Google Scholar]
- Dao T. L., Chistakos S. S., Varela R. Biochemical characterization of carcinogen-induced mammary hyperplastic aveolar nodule and tumor in the rat. Cancer Res. 1975 May;35(5):1128–1134. [PubMed] [Google Scholar]
- Gallager H. S., Martin J. E. Early phases in the development of breast cancer. Cancer. 1969 Dec;24(6):1170–1178. doi: 10.1002/1097-0142(196912)24:6<1170::aid-cncr2820240615>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., BRIZIARELLI G., SUTTON H., Jr Rapid induction of mammary carcinoma in the rat and the influence of hormones on the tumors. J Exp Med. 1959 Jan 1;109(1):25–42. doi: 10.1084/jem.109.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C., GRAND L. C., BRILLANTES F. P. Mammary cancer induced by a single feeding of polymucular hydrocarbons, and its suppression. Nature. 1961 Jan 21;189:204–207. doi: 10.1038/189204a0. [DOI] [PubMed] [Google Scholar]
- Jensen E. V. Estrogen receptors in hormone-dependent breast cancers. Cancer Res. 1975 Nov;35(11 Pt 2):3362–3364. [PubMed] [Google Scholar]
- McGuire W. L., Chamness G. C., Costlow M. E., Shepherd R. E. Hormone dependence in breast cancer. Metabolism. 1974 Jan;23(1):75–100. doi: 10.1016/0026-0495(74)90106-1. [DOI] [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. I. Incidence in BALB-c and C57BL mice. J Natl Cancer Inst. 1974 Jul;53(1):213–221. doi: 10.1093/jnci/53.1.213. [DOI] [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. VI. Tumor-producing capabilities of mammary dysplasias in BALB/cCrgl mice. J Natl Cancer Inst. 1976 Nov;57(5):1185–1189. doi: 10.1093/jnci/57.5.1185. [DOI] [PubMed] [Google Scholar]
- Medina D., Warner M. R. Mammary tumorigenesis in chemical carcinogen-treated mice. IV. Induction of mammary ductal hyperplasias. J Natl Cancer Inst. 1976 Aug;57(2):331–337. doi: 10.1093/jnci/57.2.331. [DOI] [PubMed] [Google Scholar]
- Middleton P. J. The histogenesis of mammary tumours induced in the rat by chemical carcinogens. Br J Cancer. 1965 Dec;19(4):830–839. doi: 10.1038/bjc.1965.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs B. G. Uptake of (3H)oestradiol by dimethylbenzanthracene-induced rat mammary tumours regressing spontaneously or after ovariectomy. J Endocrinol. 1969 Jul;44(3):463–464. doi: 10.1677/joe.0.0440463. [DOI] [PubMed] [Google Scholar]
- Sinha D., Dao T. L. Site of origin of mammary tumors induced by 7,12-dimethylbenz(a)anthracene in the rat. J Natl Cancer Inst. 1975 Apr;54(4):1007–1009. [PubMed] [Google Scholar]
- Warner M. R., Warner R. L. Effects of exposure of neonatal mice to 17beta-estradiol on subsequent age-incidence and morphology of carcinogen-induced mammary dysplasia. J Natl Cancer Inst. 1975 Aug;55(2):289–298. [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M., Marcum R. G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975 Aug;55(2):231–273. [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973 May;50(5):1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Mehta R. G., Boyd P. A., Goral J. E. Steroid-binding proteins of the mammary gland and their clinical significance in breast cancer. J Toxicol Environ Health Suppl. 1976;1:231–256. [PubMed] [Google Scholar]
- YOUNG S., COWAN D. M., SUTHERLAND L. E. The histology of induced mammary tumours in rats. J Pathol Bacteriol. 1963 Apr;85:331–340. doi: 10.1002/path.1700850210. [DOI] [PubMed] [Google Scholar]