FIGURE 1.
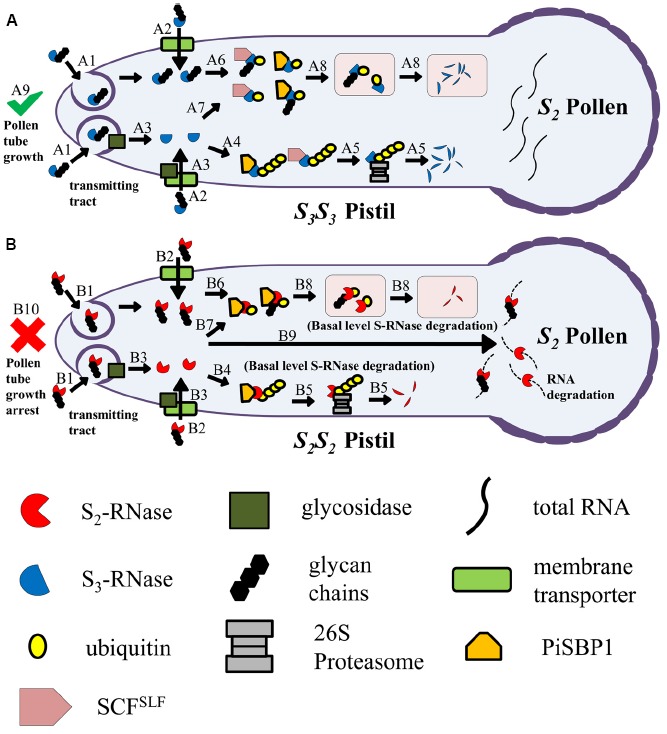
Model for uptake of S-RNase by the pollen tube in the transmitting tract of the pistil, and fates of self and non-self S-RNases after uptake. (A) An S2 pollen tube, growing in an S3S3 pistil, takes up S3-RNase (a non-self S-RNase). Two possible types of uptake mechanisms are depicted: clathrin-dependent or clathrin-independent endocytosis (A1) and membrane-transporter mediated (A2). During uptake, the N-linked glycan chains of S3-RNase may be removed by a membrane-associated glycosidase (A3). The deglycosylated S3-RNase becomes poly-ubiquitinated (A4), mediated largely by the conventional SCFSLF complex and to a much lesser extent by PiSBP1 and the PiSBP1-contaning novel SCFSLF complex (not shown). The poly-ubiquitinated S3-RNase is destined for degradation by the 26S proteasome (A5). S3-RNase may remain glycosylated and be mono-ubiquitinated (A6), again mediated largely by the conventional SCFSLF complex and to some extent by PiSBP1 and the PiSBP1-contaning novel SCFSLF complex (not shown). The deglycosylated S3-RNase may also be similarly mono-ubiquitinated (A7). The mono-ubiquitinated (deglycosylated) S3-RNase is then targeted to vacuoles or vacuole-like organelles for degradation (A8). All the steps depicted result in detoxification of the S3-RNase molecules inside the S2 pollen tube, allowing it to reach the ovary to effect fertilization (A9). (B) An S2 pollen tube, growing in an S2S2 pistil, takes up S2-RNase (self S-RNase). S2-RNase is taken up by the same mechanisms (B1 and B2) as those depicted for S3-RNase in (A), and may also be subjected to deglycoslyation (B3). None of the SLF proteins in the SCFSLF complexes are able to interact with their self S-RNase to mediate its degradation or compartmentalization. However, similar to the scenarios depicted in (A), PiSBP1 may mediate poly-ubiquitination of S2-RNase (B4) for basal-level degradation by the 26S proteasome (B5), and may mediate mono-ubiquitination of both S2-RNase (B6) and deglycosylated S2-RNase (B7). The mono-ubiquitinated (deglycosylated) S2-RNase is then targeted to vacuoles or vacuole-like organelles for degradation (B8). However, the majority of S2-RNase molecules remain intact and will degrade RNA (B9) to result in growth arrest of the pollen tube (B10). Note: To make it easier to follow the different fates of self and non-self S-RNase, S2S2 and S3S3 pistils are used in this figure; however, in nature, pistils of a self-incompatible species are normally heterozygous for the S-locus.
